Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury
- PMID: 18077691
- PMCID: PMC6673617
- DOI: 10.1523/JNEUROSCI.3489-07.2007
Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury
Abstract
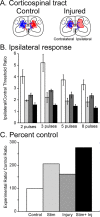
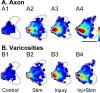
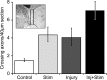
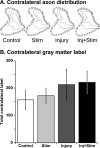
Activity-dependent competition shapes corticospinal (CS) axon outgrowth in the spinal cord during development. An important question in neural repair is whether activity can be used to promote outgrowth of CS axons in maturity. After injury, spared CS axons sprout and make new connections, but often not enough to restore function. We propose that electrically stimulating spared axons after injury will enhance sprouting and strengthen connections with spinal motor circuits. To study the effects of activity, we electrically stimulated CS tract axons in the medullary pyramid. To study the effects of injury, one pyramid was lesioned. We studied sparse ipsilateral CS projections of the intact pyramid as a model of the sparse connections preserved after CNS injury. We determined the capacity of CS axons to activate ipsilateral spinal motor circuits and traced their spinal projections. To understand the separate and combined contributions of injury and activity, we examined animals receiving stimulation only, injury only, and injury plus stimulation. Both stimulation and injury alone strengthened CS connectivity and increased outgrowth into the ipsilateral gray matter. Stimulation of spared axons after injury promoted outgrowth that reflected the sum of effects attributable to activity and injury alone. CS terminations were densest within the ventral motor territories of the cord, and connections in these animals were significantly stronger than after injury alone, indicating that activity augments injury-induced plasticity. We demonstrate that activity promotes plasticity in the mature CS system and that the interplay between activity and injury preferentially promotes connections with ventral spinal motor circuits.
Figures


Comment in
-
Strengthening corticospinal connections with chronic electrical stimulation after injury.J Neurosci. 2008 Mar 26;28(13):3262-3. doi: 10.1523/JNEUROSCI.0308-08.2008. J Neurosci. 2008. PMID: 18367593 Free PMC article. No abstract available.
References
-
- Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91:1832–1839. - PubMed
-
- Aoki M, Fujito Y, Satomi H, Kurosawa Y, Kasaba T. The possible role of collateral sprouting in the functional restitution of corticospinal connections after spinal hemisection. Neurosci Res. 1986;3:617–627. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
-
- Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
