A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish
- PMID: 18077698
- PMCID: PMC6673616
- DOI: 10.1523/JNEUROSCI.3136-07.2007
A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish
Abstract
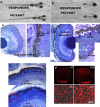
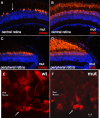
Photoreceptor degeneration is a common cause of inherited blindness worldwide. We have identified a blind zebrafish mutant with rapid degeneration of cone photoreceptors caused by a mutation in the cone phosphodiesterase c (pde6c) gene, a key regulatory component in cone phototransduction. Some rods also degenerate, primarily in areas with a low density of rods. Rod photoreceptors in areas of the retina that always have a high density of rods are protected from degeneration. Our findings demonstrate that, analogous to what happens to rod photoreceptors in the rd1 mouse model, loss of cone phosphodiesterase leads to rapid degeneration of cone photoreceptors. Furthermore, we propose that cell density plays a key role in determining whether rod photoreceptors degenerate as a secondary consequence to cone degeneration. Our zebrafish mutant serves as a model for developing therapeutic treatments for photoreceptor degeneration in humans.
Figures





References
-
- Bilotta J, Saszik S, Sutherland SE. Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev Dyn. 2001;222:564–570. - PubMed
-
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
