Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus
- PMID: 18077728
- PMCID: PMC2258705
- DOI: 10.1128/JVI.02113-07
Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus
Abstract
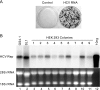
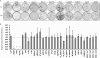
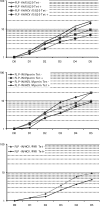
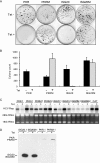
Hepatitis C virus (HCV) infection is a common cause of chronic hepatitis and is currently treated with alpha interferon (IFN-alpha)-based therapies. However, the underlying mechanism of IFN-alpha therapy remains to be elucidated. To identify the cellular proteins that mediate the antiviral effects of IFN-alpha, we created a HEK293-based cell culture system to inducibly express individual interferon-stimulated genes (ISGs) and determined their antiviral effects against HCV. By screening 29 ISGs that are induced in Huh7 cells by IFN-alpha and/or up-regulated in HCV-infected livers, we discovered that viperin, ISG20, and double-stranded RNA-dependent protein kinase (PKR) noncytolytically inhibited the replication of HCV replicons. Mechanistically, inhibition of HCV replication by ISG20 and PKR depends on their 3'-5' exonuclease and protein kinase activities, respectively. Moreover, our work, for the first time, provides strong evidence suggesting that viperin is a putative radical S-adenosyl-l-methionine (SAM) enzyme. In addition to demonstrating that the antiviral activity of viperin depends on its radical SAM domain, which contains conserved motifs to coordinate [4Fe-4S] cluster and cofactor SAM and is essential for its enzymatic activity, mutagenesis studies also revealed that viperin requires an aromatic amino acid residue at its C terminus for proper antiviral function. Furthermore, although the N-terminal 70 amino acid residues of viperin are not absolutely required, deletion of this region significantly compromises its antiviral activity against HCV. Our findings suggest that viperin represents a novel antiviral pathway that works together with other antiviral proteins, such as ISG20 and PKR, to mediate the IFN response against HCV infection.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

