Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality
- PMID: 18077788
- PMCID: PMC2265461
- DOI: 10.1182/blood-2007-06-096081
Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality
Abstract
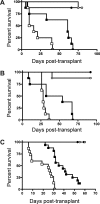
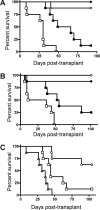
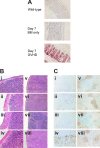
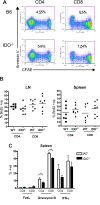
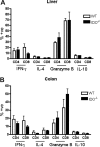
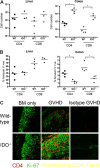
Graft-versus-host disease (GVHD) is initiated after activation of donor T cells by host antigen-presenting cells (APCs). The immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by APCs and parenchymal cells and is further inducible by inflammation. We investigated whether lethal conditioning and GVHD induce IDO and if IDO prevents tissue injury by suppressing immune responses at the induction site. We determined that IDO is a critical regulator of GVHD, most strikingly in the colon, where epithelial cells dramatically up-regulated IDO expression during GVHD. IDO(-/-) mice died more quickly from GVHD, displaying increased colonic inflammation and T-cell infiltration. GVHD protection was not mediated by control of T-cell proliferation, apoptosis, or effector mechanisms in lymphoid organs, nor did it require donor T regulatory cells. Instead, T cells in IDO(-/-) colons underwent increased proliferation and decreased apoptosis compared with their wild-type counterparts. This evidence suggests that IDO can act at the site of expression to decrease T-cell proliferation and survival, diminishing colonic inflammation and reducing disease severity. These studies are the first to identify a function for IDO in GVHD lethality and indicate that modulation of the IDO pathway may be an effective strategy for treatment of this disease.
Figures

Comment in
-
More ADO about IDO: GVHD.Blood. 2008 Mar 15;111(6):2950. doi: 10.1182/blood-2008-01-131540. Blood. 2008. PMID: 18942199 No abstract available.
References
-
- Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. - PubMed
-
- Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. - PubMed
-
- Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. - PubMed
-
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. - PubMed
-
- Ueno A, Cho S, Cheng L, et al. Transient upregulation of IDO in dendritic cells by human chorionic gonadotropin downregulates autoimmune diabetes. Diabetes. 2007;56:1686–1693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

