Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa
- PMID: 18077809
- PMCID: PMC5068549
- DOI: 10.1056/NEJMoa065711
Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa
Abstract
Background: In sub-Saharan Africa, bacterial meningitis is common and is associated with a high mortality. Adjuvant therapy with corticosteroids reduces mortality among adults in the developed world, but it has not been adequately tested in developing countries or in the context of advanced human immunodeficiency virus (HIV) infection.
Methods: We conducted a randomized, double-blind, placebo-controlled trial of dexamethasone (16 mg twice daily for 4 days) and an open-label trial of intramuscular versus intravenous ceftriaxone (2 g twice daily for 10 days) in adults with an admission diagnosis of bacterial meningitis in Blantyre, Malawi. The primary outcome was death at 40 days after randomization.
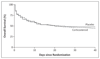
Results: A total of 465 patients, 90% of whom were HIV-positive, were randomly assigned to receive dexamethasone (233 patients) or placebo (232 patients) plus intramuscular ceftriaxone (230 patients) or intravenous ceftriaxone (235 patients). There was no significant difference in mortality at 40 days in the corticosteroid group (129 of 231 patients) as compared with the placebo group (120 of 228 patients) by intention-to-treat analysis (odds ratio, 1.14; 95% confidence interval [CI], 0.79 to 1.64) or when the analysis was restricted to patients with proven pneumococcal meningitis (68 of 129 patients receiving corticosteroids vs. 72 of 143 patients receiving placebo) (odds ratio, 1.10; 95% CI, 0.68 to 1.77). There were no significant differences between groups in the outcomes of disability and death combined, hearing impairment, and adverse events. There was no difference in mortality with intravenous ceftriaxone (121 of 230 patients) as compared with intramuscular ceftriaxone (128 of 229 patients) (odds ratio, 0.88; 95% CI, 0.61 to 1.27).
Conclusions: Adjuvant therapy with dexamethasone for bacterial meningitis in adults from an area with a high prevalence of HIV did not reduce mortality or morbidity. In this setting, intramuscular administration was not inferior to intravenous administration of ceftriaxone for bacterial meningitis. (Current Controlled Trials number, ISRCTN31371499 [controlled-trials.com].).
Copyright 2007 Massachusetts Medical Society.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Corticosteroids for acute bacterial meningitis.N Engl J Med. 2007 Dec 13;357(24):2507-9. doi: 10.1056/NEJMe0707474. N Engl J Med. 2007. PMID: 18077815 No abstract available.
-
Corticosteroids for bacterial meningitis.N Engl J Med. 2008 Mar 27;358(13):1399-400; author reply 1400-1. N Engl J Med. 2008. PMID: 18376430 No abstract available.
-
Corticosteroids for bacterial meningitis.N Engl J Med. 2008 Mar 27;358(13):1400; author reply 1400-1. N Engl J Med. 2008. PMID: 18376431 No abstract available.
-
Adjunctive corticosteroids for adults in sub-Saharan Africa with bacterial meningitis.Curr Infect Dis Rep. 2008 Jul;10(4):290-1. Curr Infect Dis Rep. 2008. PMID: 18765107 No abstract available.
References
-
- Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32:675–85. - PubMed
-
- Murray CJ, Lopez AD, editors. Global burden of disease and injury series. Vol 2, Global health statistics. Geneva: World Health Organization; 1996.
-
- Gordon SB, Walsh AL, Chaponda M, et al. Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clin Infect Dis. 2000;31:53–7. - PubMed
-
- van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical