Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes
- PMID: 18077827
- PMCID: PMC2444000
- DOI: 10.1194/jlr.R700018-JLR200
Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes
Abstract
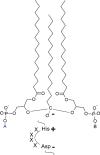
In this article, the formation of prokaryotic and eukaryotic cardiolipin is reviewed in light of its biological function. I begin with a detailed account of the structure of cardiolipin, its stereochemistry, and the resulting physical properties, and I present structural analogs of cardiolipin that occur in some organisms. Then I continue to discuss i) the de novo formation of cardiolipin, ii) its acyl remodeling, iii) the assembly of cardiolipin into biological membranes, and iv) the degradation of cardiolipin, which may be involved in apoptosis and mitochondrial fusion. Thus, this article covers the entire metabolic cycle of this unique phospholipid. It is shown that mitochondria produce cardiolipin species with a high degree of structural uniformity and molecular symmetry, among which there is often a dominant form with four identical acyl chains. The subsequent assembly of cardiolipin into functional membranes is largely unknown, but the analysis of crystal structures of membrane proteins has revealed a first glimpse into the underlying principles of cardiolipin-protein interactions. Disturbances of cardiolipin metabolism are crucial in the pathophysiology of human Barth syndrome and perhaps also play a role in diabetes and ischemic heart disease.
Figures






References
-
- LeCocq J., and C. E. Ballou. 1964. On the structure of cardiolipin. Biochemistry. 3 976–980. - PubMed
-
- Powell G. L., and J. Jacobus. 1974. The nonequivalence of the phosphorus atoms in cardiolipin. Biochemistry. 13 4024–4026. - PubMed
-
- Schlame M., M. Ren, Y. Xu, M. L. Greenberg, and I. Haller. 2005. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids. 138 38–49. - PubMed
-
- Kates M., J-Y. Syz, D. Gosser, and T. H. Haines. 1993. pH-dissociation characteristics of cardiolipin and its 2′-deoxy analogue. Lipids. 28 877–882. - PubMed
-
- Dahlberg M. 2007. Polymorphic phase behavior of cardiolipin derivatives studied by coarse-grained molecular dynamics. J. Phys. Chem. 111 7194–7200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

