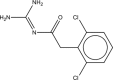
Guanfacine and guanfacine extended release: treatment for ADHD and related disorders
- PMID: 18078429
- PMCID: PMC6494159
- DOI: 10.1111/j.1527-3458.2007.00026.x
Guanfacine and guanfacine extended release: treatment for ADHD and related disorders
Abstract
Guanfacine, an alpha(2A) adrenoceptor agonist, is U.S. Food and Drug Administration (FDA)-approved for the treatment of hypertension in adolescents and adults. It also has been used "off-label" for several years in children as a possible treatment for attention-deficit/hyperactivity disorder (ADHD) and pervasive developmental disorders (PDDs). Small placebo-controlled trials support the use of guanfacine for the treatment of ADHD. There is more limited research on the use of guanfacine in treating hyperactivity occurring in children diagnosed with PDD. Recently, guanfacine extended release (GXR), a once-daily formulation has been manufactured and studied in phase III clinical trials. Based on preliminary scientific presentations, it also appears to be efficacious in improving ADHD in children. The most common adverse effects associated with guanfacine and GXR treatment is sedation. Adverse cardiovascular effects are uncommon, although modest reductions in blood pressure and heart rate are common. If GXR is FDA-approved, it would be the first alpha(2A) adrenoceptor agonist marketed for ADHD.
Similar articles
-
Guanfacine extended release: a novel treatment for attention-deficit/hyperactivity disorder in children and adolescents.Clin Ther. 2013 Nov;35(11):1778-93. doi: 10.1016/j.clinthera.2013.09.005. Epub 2013 Oct 16. Clin Ther. 2013. PMID: 24139092 Review.
-
Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial.J Am Acad Child Adolesc Psychiatry. 2009 Feb;48(2):155-65. doi: 10.1097/CHI.0b013e318191769e. J Am Acad Child Adolesc Psychiatry. 2009. PMID: 19106767 Clinical Trial.
-
Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration.J Am Acad Child Adolesc Psychiatry. 2013 Sep;52(9):921-30. doi: 10.1016/j.jaac.2013.06.006. Epub 2013 Aug 1. J Am Acad Child Adolesc Psychiatry. 2013. PMID: 23972694 Clinical Trial.
-
Guanfacine extended release in the treatment of attention deficit hyperactivity disorder in children and adolescents.Drugs Today (Barc). 2010 May;46(5):299-314. doi: 10.1358/dot.2010.46.5.1450095. Drugs Today (Barc). 2010. PMID: 20517532 Review.
-
A Randomized, Placebo-Controlled Trial of Guanfacine Extended Release in Adolescents With Attention-Deficit/Hyperactivity Disorder.J Am Acad Child Adolesc Psychiatry. 2015 Nov;54(11):916-25.e2. doi: 10.1016/j.jaac.2015.08.016. Epub 2015 Sep 15. J Am Acad Child Adolesc Psychiatry. 2015. PMID: 26506582 Clinical Trial.
Cited by
-
Efficacy and safety of guanfacine in hospitalized patients with delirium: A scoping review.Crit Care Resusc. 2024 Nov 24;26(4):286-294. doi: 10.1016/j.ccrj.2024.08.009. eCollection 2024 Dec. Crit Care Resusc. 2024. PMID: 39781496 Free PMC article.
-
Development of the Korean Practice Parameter for Adult Attention-Deficit/Hyperactivity Disorder.Soa Chongsonyon Chongsin Uihak. 2020 Jan 1;31(1):5-25. doi: 10.5765/jkacap.190030. Soa Chongsonyon Chongsin Uihak. 2020. PMID: 32612409 Free PMC article.
-
Norepinephrine and stimulant addiction.Addict Biol. 2009 Apr;14(2):119-29. doi: 10.1111/j.1369-1600.2008.00138.x. Epub 2008 Sep 22. Addict Biol. 2009. PMID: 18811678 Free PMC article. Review.
-
Nonstimulant Medications for Attention-Deficit/Hyperactivity Disorder (ADHD) in Adults: Systematic Review and Meta-analysis.CNS Drugs. 2023 May;37(5):381-397. doi: 10.1007/s40263-023-01005-8. Epub 2023 May 11. CNS Drugs. 2023. PMID: 37166701
-
Anxiety and school avoidance in an 8-year-old child with epilepsy.Epilepsy Behav Rep. 2024 Mar 13;26:100659. doi: 10.1016/j.ebr.2024.100659. eCollection 2024. Epilepsy Behav Rep. 2024. PMID: 38532902 Free PMC article.
References
-
- Abo‐Zena RA, Bobek MB, Dweik RA (2000) Hypertensive urgency induced by an interaction of mirtazapine and clonidine. Pharmacotherapy 20:76‐78. - PubMed
-
- Aman MG, Singh NN, Stewart AW, Field CJ (1985) The aberrant behavior checklist: A behavior rating scale of the assessment of treatment effects. Am J Ment Deficiency 89:485‐491. - PubMed
-
- Arnsten AF, Li BM (2005) Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377‐1184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical