Glucuronidation and sulfonation, in vitro, of the major endocrine-active metabolites of methoxychlor in the channel catfish, Ictalurus punctatus, and induction following treatment with 3-methylcholanthrene
- PMID: 18078677
- PMCID: PMC2268215
- DOI: 10.1016/j.aquatox.2007.11.003
Glucuronidation and sulfonation, in vitro, of the major endocrine-active metabolites of methoxychlor in the channel catfish, Ictalurus punctatus, and induction following treatment with 3-methylcholanthrene
Abstract
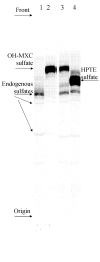
The organochlorine pesticide, methoxychlor (MXC), is metabolized in animals to phenolic mono- and bis-demethylated metabolites (OH-MXC and HPTE, respectively) that interact with estrogen receptors and may be endocrine disruptors. The phase II detoxication of these compounds will influence the duration of action of the estrogenic metabolites, but has not been investigated extensively. In this study, the glucuronidation and sulfonation of OH-MXC and HPTE were investigated in subcellular fractions of liver and intestine from untreated, MXC-treated and 3-methylcholanthrene (3-MC)-treated channel catfish, Ictalurus punctatus. MXC-treated fish were given i.p. injections of 2mg MXC/kg daily for 6 days and sacrificed 24h after the last dose. The 3-MC treatment was a single 10mg/kg i.p. dose 5 days prior to sacrifice. In hepatic microsomes from control fish, the V(max) value (mean+/-S.D., n=4) for glucuronidation of OH-MXC was 270+/-50pmol/min/mg protein, higher than found for HPTE (110+/-20pmol/min/mg protein). For each substrate, the V(max) values observed in intestinal microsomes were approximately twice those found in the liver. The K(m) values for OH-MXC and HPTE glucuronidation in control liver were not significantly different and were 0.32+/-0.04mM for OH-MXC and 0.26+/-0.06mM for HPTE. The K(m) for the co-substrate, UDPGA, was higher in liver (0.28+/-0.09mM) than intestine (0.04+/-0.02mM). Treatment with 3-MC but not MXC increased the V(max) for glucuronidation in liver and intestine. Glucuronidation was a more efficient pathway than sulfonation for both substrates, in both tissues. The V(max) values for sulfonation of OH-MXC and HPTE, respectively, in liver cytosol were 7+/-3 and 17+/-4pmol/min/mg protein and in intestinal cytosol were 13+/-3 and 30+/-5pmol/min/mg protein. Treatment with 3-MC but not MXC increased rates of sulfonation of OH-MXC and HPTE and the model substrate, 3-hydroxy-benzo(a)pyrene in both intestine and liver. Comparison of the kinetics of the conjugation pathways with those published for the demethylation of MXC showed that formation of the endocrine-active metabolites was more efficient than either conjugation pathway. Residues of OH-MXC and HPTE were detected in extracts of liver microsomes from MXC-treated fish. This work showed that although OH-MXC and HPTE could be eliminated by glucuronidation and sulfonation, the phase II pathways were less efficient than the phase I pathway leading to formation of these endocrine-active metabolites.
Figures





References
-
- Andersson T, Pesonen M, Johansson C. Differential induction of cytochrome P-450-dependent monooxygenase, epoxide hydrolase, glutathione transferase and UDP glucuronosyl transferase activities in the liver of the rainbow trout by beta-naphthoflavone or Clophen A50. Biochem Pharmacol. 1985;34:3309–3314. - PubMed
-
- Bock KW, Lipp HP, Bock-Hennig BS. Induction of drug-metabolizing enzymes by xenobiotics. Xenobiotica. 1990;20:1101–1111. - PubMed
-
- Campoy C, Jimenez M, Olea-Serrano MF, Moreno-Frias M, Canabate F, Olea N, Bayes R, Molina-Font JA. Analysis of organochlorine pesticides in human milk: preliminary results. Early Hum Dev. 2001;65(Suppl):S183–190. - PubMed
-
- Clarke DJ, Burchell B, George SG. Differential expression and induction of UDP-glucuronosyltransferase isoforms in hepatic and extrahepatic tissues of a fish, Pleuronectes platessa: immunochemical and functional characterization. Toxicol Appl Pharmacol. 1992;115:130–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

