Uncovering the mechanisms of estrogen effects on hippocampal function
- PMID: 18078984
- PMCID: PMC2440702
- DOI: 10.1016/j.yfrne.2007.08.006
Uncovering the mechanisms of estrogen effects on hippocampal function
Abstract
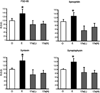
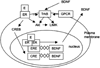
Estrogens have direct effects on the brain areas controlling cognition. One of the most studied of these regions is the dorsal hippocampal formation, which governs the formation of spatial and episodic memories. In laboratory animals, most investigators report that estrogen enhances synaptic plasticity and improves performance on hippocampal-dependent cognitive behaviors. This review summarizes work conducted in our laboratory and others toward identifying estrogen's actions in the hippocampal formation, and the mechanisms for these actions. Physiologic and pharmacologic estrogen affects cognitive behavior in mammals, which may be applicable to human health and disease. The effects of estrogen in the hippocampal formation that lead to modulation of hippocampal function include effects on cell morphology, synapse formation, signaling, and excitability that have been studied in laboratory mice, rats, and primates. Finally, estrogen may signal through both nuclear and extranuclear hippocampal estrogen receptors to achieve its downstream effects.
Figures




References
-
- Nelson RJ. An Introduction to Behavioral Endocrinology. sunderland, MA: sinauer associates; 2005.
-
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. - PubMed
-
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. - PubMed
-
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

