Forkhead box protein O1 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components
- PMID: 18079193
- PMCID: PMC2275355
- DOI: 10.1210/en.2007-1470
Forkhead box protein O1 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components
Abstract
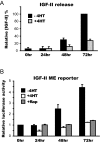
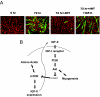
The forkhead transcription factor forkhead box protein O1 (FoxO1), a downstream target of phosphatidylinositol 3-kinase/Akt signaling, has been reported to suppress skeletal myocyte differentiation, but the mechanism by which FoxO1 regulates myogenesis is not fully understood. We have previously demonstrated that a nutrient-sensing mammalian target of rapamycin (mTOR) pathway controls the autocrine production of IGF-II and the subsequent phosphatidylinositol 3-kinase/Akt signaling downstream of IGF-II in myogenesis. Here we report a regulatory loop connecting FoxO1 to the mTOR pathway. Inducible activation of a FoxO1 active mutant in the C2C12 mouse myoblasts blocks myogenic differentiation at an early stage and meanwhile leads to proteasome-dependent degradation of a specific subset of components in the mTOR signaling network, including mTOR, raptor, tuberous sclerosis complex 2, and S6 protein kinase 1. This function of FoxO1 requires new protein synthesis, consistent with the idea that a transcriptional target of FoxO1 may be responsible for the degradation of mTOR. We further show that active FoxO1 inhibits IGF-II expression at the transcriptional activation level, through the modulation of mTOR protein levels. Moreover, the addition of exogenous IGF-II fully rescues myocyte differentiation from FoxO inhibition. Taken together, we propose that the mTOR-IGF-II pathway is a major mediator of FoxO's inhibitory function in skeletal myogenesis.
Figures




References
-
- Weintraub H 1993 The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75:1241–1244 - PubMed
-
- Naya FS, Olson E 1999 MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11:683–688 - PubMed
-
- Florini JR, Ewton DZ, Magri KA 1991 Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol 53:201–216 - PubMed
-
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N 2001 Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

