Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons
- PMID: 18079199
- PMCID: PMC2274903
- DOI: 10.1210/en.2007-0942
Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons
Abstract
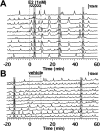
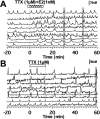
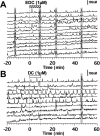
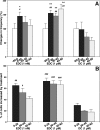
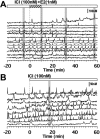
Feedback controls of estrogen in LHRH-1 neurons play a pivotal role in reproductive function. However, the mechanism of estrogen action in LHRH-1 neurons is still unclear. In the present study, the effect of estrogens on intracellular calcium ([Ca(2+)](i)) oscillations in primate LHRH-1 neurons was examined. Application of 17beta-estradiol (E(2), 1 nm) for 10 min increased the frequency of [Ca(2+)](i) oscillations within a few minutes. E(2) also increased the frequency of [Ca(2+)](i) synchronization among LHRH-1 neurons. Similar E(2) effects on the frequency of [Ca(2+)](i) oscillations were observed under the presence of tetrodotoxin, indicating that estrogen appears to cause direct action on LHRH-1 neurons. Moreover, application of a nuclear membrane-impermeable estrogen dendrimer conjugate, not control dendrimer, resulted in a robust increase in the frequencies of [Ca(2+)](i) oscillations and synchronizations, indicating that effects estrogens on [Ca(2+)](i) oscillations and their synchronizations do not require their entry into the cell nucleus. Exposure of cells to E(2) in the presence of the estrogen receptor antagonist ICI 182,780 did not change the E(2)-induced increase in the frequency of [Ca(2+)](i) oscillations or the E(2)-induced increase in the synchronization frequency. Collectively, estrogens induce rapid, direct stimulatory actions through receptors located in the cell membrane/cytoplasm of primate LHRH-1 neurons, and this action of estrogens is mediated by an ICI 182,780-insensitive mechanism yet to be identified.
Figures
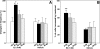
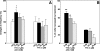
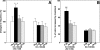
Comment in
-
Unique estrogenic mechanisms for unique gonadotropin-releasing hormone neurons?Endocrinology. 2008 Nov;149(11):5325-7. doi: 10.1210/en.2008-1231. Endocrinology. 2008. PMID: 18936493 Free PMC article. No abstract available.
References
-
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW 1983 Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 304:345–347 - PubMed
-
- Lehman MN, Karsch FJ 1993 Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133:887–895 - PubMed
-
- Herbison AE, Horvath TL, Naftolin F, Leranth C 1995 Distribution of estrogen receptor-immunoreactive cells in monkey hypothalamus: relationship to neurones containing luteinizing hormone-releasing hormone and tyrosine hydroxylase. Neuroendocrinology 61:1–10 - PubMed
-
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 - PubMed
-
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1–7 GnRH neurons. Endocrinology 140:5045–5053 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

