Nitric oxide regulates neutrophil migration through microparticle formation
- PMID: 18079439
- PMCID: PMC2189628
- DOI: 10.2353/ajpath.2008.070069
Nitric oxide regulates neutrophil migration through microparticle formation
Abstract
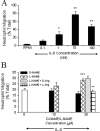
The role of nitric oxide (NO) in regulating neutrophil migration has been investigated. Human neutrophil migration to interleukin (IL)-8 (1 nmol/L) was measured after a 1-hour incubation using a 96-well chemotaxis plate assay. The NO synthase inhibitor N(G)-nitro-l-arginine methyl ester (L-NAME) significantly (P < 0.001) enhanced IL-8-induced migration by up to 45%. Anti-CD18 significantly (P < 0.001) inhibited both IL-8-induced and L-NAME enhanced migration. Antibodies to L-selectin or PSGL-1 had no effect on IL-8-induced migration but prevented the increased migration to IL-8 induced by L-NAME. L-NAME induced generation of neutrophil-derived microparticles that was significantly (P < 0.01) greater than untreated neutrophils or D-NAME. This microparticle formation was dependent on calpain activity and superoxide production. Only microparticles from L-NAME and not untreated or D-NAME-treated neutrophils induced a significant (P < 0.01) increase in IL-8-induced migration and transendothelial migration. Pretreatment of microparticles with antibodies to L-selectin (DREG-200) or PSGL-1 (PL-1) significantly (P < 0.001) inhibited this effect. The ability of L-NAME-induced microparticles to enhance migration was found to be dependent on the number of microparticles produced and not an increase in microparticle surface L-selectin or PSGL-1 expression. These data show that NO can modulate neutrophil migration by regulating microparticle formation.
Figures




References
-
- Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. - PubMed
-
- Lukacs NW, Ward PA. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–304. - PubMed
-
- Malik AB, Lo SK. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol Rev. 1996;48:213–229. - PubMed
-
- Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. - PubMed
-
- Kakkar AK, Lefer DJ. Leukocyte and endothelial adhesion molecule studies in knockout mice. Curr Opin Pharmacol. 2004;4:154–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

