Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice
- PMID: 18079494
- PMCID: PMC2277210
- DOI: 10.1164/rccm.200708-1233OC
Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice
Abstract
Rationale: Chronic obstructive pulmonary disease is a leading cause of death worldwide, but its pathogenesis is not well understood. Previous studies have shown that airway surface dehydration in beta-epithelial Na(+) channel (betaENaC)-overexpressing mice caused a chronic lung disease with high neonatal pulmonary mortality and chronic bronchitis in adult survivors.
Objectives: The aim of this study was to identify the initiating lesions and investigate the natural progression of lung disease caused by airway surface dehydration.
Methods: Lung morphology, gene expression, bronchoalveolar lavage, and lung mechanics were studied at different ages in betaENaC-overexpressing mice.
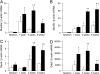
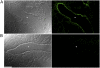
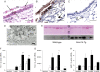
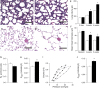
Measurements and main results: Mucus obstruction in betaENaC-overexpressing mice originated in the trachea in the first days of life and was associated with hypoxia, airway epithelial necrosis, and death. In surviving betaENaC-overexpressing mice, mucus obstruction extended into the lungs and was accompanied by goblet cell metaplasia, increased mucin expression, and airway inflammation with transient perinatal increases in tumor necrosis factor-alpha and macrophages, IL-13 and eosinophils, and persistent increases in keratinocyte-derived cytokine (KC), neutrophils, and chitinases in the lung. betaENaC-overexpressing mice also developed emphysema with increased lung volumes, distal airspace enlargement, and increased lung compliance.
Conclusions: Our studies demonstrate that airway surface dehydration is sufficient to initiate persistent neutrophilic airway inflammation with chronic airways mucus obstruction and to cause transient eosinophilic airway inflammation and emphysema. These results suggest that deficient airway surface hydration may play a critical role in the pathogenesis of chronic obstructive pulmonary diseases of different etiologies and serve as a target for novel therapies.
Figures





References
-
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–1080. - PubMed
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, and Valle D, editors. The metabolic and molecular bases of inherited disease, 8th ed. New York: McGraw-Hill; 2001. pp. 5121–5188.
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 1991;253:202–205. - PubMed
-
- Canessa CM, Schild L, Buell G, Thorens B, Gautschl I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994;367:463–467. - PubMed
-
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 1995;269:847–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

