Intrasplicing coordinates alternative first exons with alternative splicing in the protein 4.1R gene
- PMID: 18079699
- PMCID: PMC2206138
- DOI: 10.1038/sj.emboj.7601957
Intrasplicing coordinates alternative first exons with alternative splicing in the protein 4.1R gene
Abstract
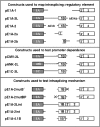
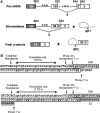
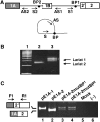
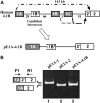
In the protein 4.1R gene, alternative first exons splice differentially to alternative 3' splice sites far downstream in exon 2'/2 (E2'/2). We describe a novel intrasplicing mechanism by which exon 1A (E1A) splices exclusively to the distal E2'/2 acceptor via two nested splicing reactions regulated by novel properties of exon 1B (E1B). E1B behaves as an exon in the first step, using its consensus 5' donor to splice to the proximal E2'/2 acceptor. A long region of downstream intron is excised, juxtaposing E1B with E2'/2 to generate a new composite acceptor containing the E1B branchpoint/pyrimidine tract and E2 distal 3' AG-dinucleotide. Next, the upstream E1A splices over E1B to this distal acceptor, excising the remaining intron plus E1B and E2' to form mature E1A/E2 product. We mapped branchpoints for both intrasplicing reactions and demonstrated that mutation of the E1B 5' splice site or branchpoint abrogates intrasplicing. In the 4.1R gene, intrasplicing ultimately determines N-terminal protein structure and function. More generally, intrasplicing represents a new mechanism by which alternative promoters can be coordinated with downstream alternative splicing.
Figures





References
-
- Baklouti F, Huang SC, Vulliamy TJ, Delaunay J, Benz EJ Jr (1997) Organization of the human protein 4.1 genomic locus: new insights into the tissue-specific alternative splicing of the pre-mRNA. Genomics 39: 289–302 - PubMed

