The biology and future prospects of antivirulence therapies
- PMID: 18079741
- PMCID: PMC2211378
- DOI: 10.1038/nrmicro1818
The biology and future prospects of antivirulence therapies
Erratum in
- Nat Rev Microbiol. 2009 Nov;7(11):836
Abstract
The emergence and increasing prevalence of bacterial strains that are resistant to available antibiotics demand the discovery of new therapeutic approaches. Targeting bacterial virulence is an alternative approach to antimicrobial therapy that offers promising opportunities to inhibit pathogenesis and its consequences without placing immediate life-or-death pressure on the target bacterium. Certain virulence factors have been shown to be potential targets for drug design and therapeutic intervention, whereas new insights are crucial for exploiting others. Targeting virulence represents a new paradigm to empower the clinician to prevent and treat infectious diseases.
Figures
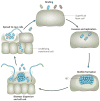

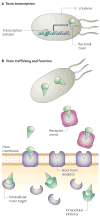
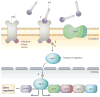
References
-
- Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. - PubMed
-
- Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis. 2005;5:450–459. - PubMed
-
- Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293:1786–1790. - PubMed
-
- Vicente M, et al. The fallacies of hope: will we discover new antibiotics to combat pathogenic bacteria in time? FEMS Microbiol Rev. 2006;30:841–852. - PubMed
-
- Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nature Med. 2004;10:S122–S129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

