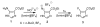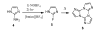Photochemical Schiemann Reaction in Ionic Liquids
- PMID: 18079989
- PMCID: PMC2136405
- DOI: 10.1016/j.jfluchem.2007.03.012
Photochemical Schiemann Reaction in Ionic Liquids
Abstract
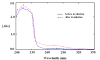
Photochemical Schiemann reactions of imidazole derivatives 1 and 4 were carried out in 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid [bmim][BF(4)] as solvent. The effects of temperature, co-solvent and wavelength on the rate of the reaction and product yield were examined. The use of ionic liquid increases the yield of the photochemical fluorodediazoniation reaction of 2 at 0°C. Careful temperature control is necessary to minimize the photodecomposition of the ionic liquid in order to increase the yield of product.
Figures
References
-
- Balz G, Schiemann G. Chem Ber. 1927;60:1186.
-
- Langlois B. In: Methods of Organic Chemistry (Houben-Weyl). Organo-Fluorine Compounds. Basner B, Hagemann H, Tatlow JC, editors. 10a. Thieme; Stuttgart: 2000. pp. 686–740.
-
- Kirk KL, Cohen LA. In: Biochemistry of the Carbon-Fluorine Bond, ACS Symposium Series. Filler R, editor. 1976. pp. 23–36.
-
- Milanesi A, Farnoni M, Albini A. J Org Chem. 2005;70:603–610. - PubMed
- Canning PSJ, McCrudden K, Maskill H, Sexton B. J Chem Soc Perkin Trans. 1999;2:2735–2740.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous