Regulation of cell-matrix contacts and beta-catenin signaling in VSMC by integrin-linked kinase: implications for intimal thickening
- PMID: 18080083
- PMCID: PMC2853711
- DOI: 10.1007/s00395-007-0693-9
Regulation of cell-matrix contacts and beta-catenin signaling in VSMC by integrin-linked kinase: implications for intimal thickening
Abstract
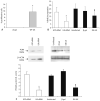
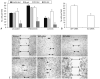
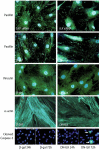
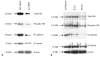
Vascular smooth muscle cell (VSMC) proliferation and migration is responsible for intimal thickening that occurs in restenosis and atherosclerosis. Integrin-linked kinase (ILK) is a serine/threonine protein kinase implicated in signaling pathways involved in cell proliferation and migration. We studied the involvement of ILK in intimal thickening. ILK expression was significantly increased in two models of intimal thickening: balloon-injured rat carotid arteries and human saphenous vein organ cultures. Over-expression of a dominant negative ILK (DN-ILK) significantly reduced intimal thickening by approximately 50% in human saphenous vein organ cultures, demonstrating an important role in intimal thickening. ILK protein and activity was reduced on laminin and up-regulated on fibronectin, indicating ILK protein expression is modulated by extracellular matrix composition. Inhibition of ILK by siRNA knockdown and DN-ILK significantly decreased VSMC proliferation and migration while wild type ILK significantly increased proliferation and migration on laminin, confirming an essential role of ILK in both processes. Localization of paxillin and vinculin and protein levels of FAK and phospho-FAK indicated that inhibition of ILK reduced focal adhesion formation. Additionally, inhibition of ILK significantly attenuated the presence of the cell-cell complex proteins N-cadherin and beta-catenin, and beta-catenin signaling. We therefore suggest ILK modulates VSMC proliferation and migration at least in part by acting as a molecular scaffold in focal adhesions as well as modulating the stability of cell-cell contact proteins and beta-catenin signaling. In summary, ILK plays an important role in intimal thickening by modulating VSMC proliferation and migration via regulation of cell-matrix and cell-cell contacts and beta-catenin signaling.
Figures



References
-
- Bar H, Wende P, Watson L, Denger S, Van Eys G, Kreuzer J, Jahn L. Smoothelin is an indicator of reversible phenotype modulation of smooth muscle cells in balloon-injured rat carotid arteries. Basic Res Cardiol. 2002;97:9–16. - PubMed
-
- Chen X, Gumbiner BM. Cross-talk between different adhesion molecules. Curr Opin Cell Biol. 2006;18:572–578. - PubMed
-
- Dietrich T, Perlitz C, Licha K, Stawowy P, Atrott K, Tachezy M, Meyborg H, Stocker C, Grafe M, Fleck E, Schirner M, Graf K. ED-B fibronectin (ED-B) can be targeted using a novel single chain antibody conjugate and is associated with macrophage accumulation in atherosclerotic lesions. Basic Res Cardiol. 2007;102:298–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

