Towards the optimal screening collection: a synthesis strategy
- PMID: 18080276
- PMCID: PMC2645036
- DOI: 10.1002/anie.200703073
Towards the optimal screening collection: a synthesis strategy
Abstract
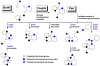
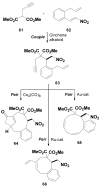
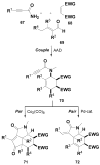
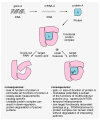
The development of effective small-molecule probes and drugs entails at least three stages: 1) a discovery phase, often requiring the synthesis and screening of candidate compounds, 2) an optimization phase, requiring the synthesis and analysis of structural variants, 3) and a manufacturing phase, requiring the efficient, large-scale synthesis of the optimized probe or drug. Specialized project groups tend to undertake the individual activities without prior coordination; for example, contracted (outsourced) chemists may perform the first activity while in-house medicinal and process chemists perform the second and third development stages, respectively. The coordinated planning of these activities in advance of the first small-molecule screen tends not to be undertaken, and each project group can encounter a bottleneck that could, in principle, have been avoided with advance planning. Therefore, a challenge for synthetic chemistry is to develop a new kind of chemistry that yields a screening collection comprising small molecules that increase the probability of success in all three phases. Although this transformative chemistry remains elusive, progress is being made. Herein, we review a newly emerging strategy in diversity-oriented small-molecule synthesis that may have the potential to achieve these challenging goals.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

