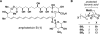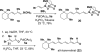Simple, efficient, and modular syntheses of polyene natural products via iterative cross-coupling
- PMID: 18081295
- PMCID: PMC3107126
- DOI: 10.1021/ja078129x
Simple, efficient, and modular syntheses of polyene natural products via iterative cross-coupling
Abstract
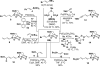
This communication describes the discovery of air-stable and highly versatile B-protected haloalkenylboronic acid building blocks for iterative cross-coupling. These reagents enable the total synthesis of polyene natural products with extraordinary levels of simplicity, efficiency, and modularity. Specifically, all-trans-retinal, β-parinaric acid, and one-half the amphotericin B macrolide skeleton were prepared using only the Suzuki-Miyaura reaction in an iterative manner to bring together collections of simple and readily-accessible building blocks. In contrast to their boronic acid counterparts, the intermediate polyenylboronate esters are remarkably stable (to both column purification and storage), which is critical to their successful utilization. Moreover, the reactive boronic acids can be cleanly liberated using very mild aqueous base. These advances have enabled preparation of the longest polyene ever synthesized using the SM reaction. We additionally report, to the best of our knowledge, the first triply metal selective (Zn vs. Sn and B) cross-coupling reaction, the first selective cross-coupling with a differentially-ligated diboron reagent, and the first cross-couplings between polyenylchlorides and vinylboronic acids. Collectively, these new building blocks and methods can dramatically improve the way polyene natural products and their derivatives are synthesized in the laboratory.
Figures
References
-
- Corey EJ, Czakó B, Kürti L. Molecules and Medicine. Wiley; New Jersey: 2007.
-
- Thirsk C, Whiting A. J. Chem. Soc. Perkin Trans. 2002;1:999–1023.
-
- Ermishkin LN, Kasumov Kh.M., Potzeluyev VM. Nature. 1976;262:698–699. - PubMed
- Hartsel SD, Hatch C, Ayenew W. J. Liposome Res. 1993;3:377–408.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources