Chemical interplay in the mechanism of partial agonist activation in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
- PMID: 18081322
- PMCID: PMC2212281
- DOI: 10.1021/bi702004b
Chemical interplay in the mechanism of partial agonist activation in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
Abstract
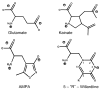
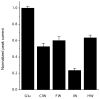
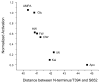
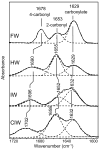
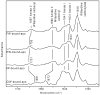
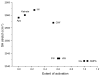
Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, one subtype in the family of ionotropic glutamate receptors, are the main receptors responsible for excitatory signaling in the mammalian central nervous system. Previous studies utilitizing the isolated ligand binding domain of these receptors have provided insight into the role of specific ligand-protein interactions in mediating receptor activation. However, these studies relied heavily on the partial agonist kainate, in which the alpha-amine group is constrained in a pyrrolidine ring. Here we have studied a series of substituted and unsubstituted willardiines with primary alpha-amine groups similar to that of the full agonist glutamate whose activation can be varied depending on the size of the substituent. The specific ligand-protein interactions in the mechanism of partial agonism in this subtype were investigated using vibrational spectroscopy, and the large-scale conformational changes in the ligand binding domain were studied with fluorescence resonance energy transfer (FRET). These investigations show that the strength of the interaction at the alpha-amine group correlates with the extent of cleft closure and extent of activation, with the agonist of higher efficacy showing larger cleft closure and stronger interactions at this group, suggesting that this is one of the mechanisms by which the agonist controls receptor activation.
Figures

Similar articles
-
Role of the chemical interactions of the agonist in controlling alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activation.Biochemistry. 2007 Feb 6;46(5):1343-9. doi: 10.1021/bi062270l. Biochemistry. 2007. PMID: 17260963 Free PMC article.
-
Probing the function, conformational plasticity, and dimer-dimer contacts of the GluR2 ligand-binding core: studies of 5-substituted willardiines and GluR2 S1S2 in the crystal.Biochemistry. 2003 May 13;42(18):5201-13. doi: 10.1021/bi020632t. Biochemistry. 2003. PMID: 12731861
-
Role of conformational dynamics in α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor partial agonism.J Biol Chem. 2012 Dec 21;287(52):43557-64. doi: 10.1074/jbc.M112.371815. Epub 2012 Oct 31. J Biol Chem. 2012. PMID: 23115239 Free PMC article.
-
Glutamate receptors as seen by light: spectroscopic studies of structure-function relationships.Braz J Med Biol Res. 2007 Nov;40(11):1419-27. doi: 10.1590/s0100-879x2007001100001. Braz J Med Biol Res. 2007. PMID: 17934637 Free PMC article. Review.
-
The biochemistry, ultrastructure, and subunit assembly mechanism of AMPA receptors.Mol Neurobiol. 2010 Dec;42(3):161-84. doi: 10.1007/s12035-010-8149-x. Epub 2010 Nov 16. Mol Neurobiol. 2010. PMID: 21080238 Free PMC article. Review.
Cited by
-
Molecular Dynamics Investigation of gluazo, a Photo-Switchable Ligand for the Glutamate Receptor GluK2.PLoS One. 2015 Aug 26;10(8):e0135399. doi: 10.1371/journal.pone.0135399. eCollection 2015. PLoS One. 2015. PMID: 26308344 Free PMC article.
-
Vibrational spectroscopic investigation of the ligand binding domain of kainate receptors.Protein Sci. 2009 Aug;18(8):1585-91. doi: 10.1002/pro.174. Protein Sci. 2009. PMID: 19544581 Free PMC article.
-
Subunit arrangement in N-methyl-D-aspartate (NMDA) receptors.J Biol Chem. 2010 May 14;285(20):15296-15301. doi: 10.1074/jbc.M109.085035. Epub 2010 Mar 19. J Biol Chem. 2010. PMID: 20304927 Free PMC article.
-
Structural dynamics of the glycine-binding domain of the N-methyl-D-aspartate receptor.J Biol Chem. 2015 Jan 9;290(2):797-804. doi: 10.1074/jbc.M114.605436. Epub 2014 Nov 17. J Biol Chem. 2015. PMID: 25404733 Free PMC article.
-
NMR spectroscopy of the ligand-binding core of ionotropic glutamate receptor 2 bound to 5-substituted willardiine partial agonists.J Mol Biol. 2008 May 2;378(3):673-85. doi: 10.1016/j.jmb.2008.03.012. Epub 2008 Mar 14. J Mol Biol. 2008. PMID: 18387631 Free PMC article.
References
-
- Oswald RE. Ionotropic glutamate receptor recognition and activation. Adv Protein Chem. 2004;68:313–349. - PubMed
-
- McFeeters RL, Oswald RE. Emerging structural explanations of ionotropic glutamate receptor function. Faseb J. 2004;18:428–438. - PubMed
-
- Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3:91–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

