Antimalarial quinolones: synthesis, potency, and mechanistic studies
- PMID: 18082162
- PMCID: PMC2323441
- DOI: 10.1016/j.exppara.2007.10.016
Antimalarial quinolones: synthesis, potency, and mechanistic studies
Abstract
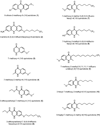
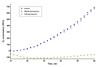
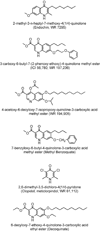
In the present article we examine the antiplasmodial activities of novel quinolone derivatives bearing extended alkyl or alkoxy side chains terminated by a trifluoromethyl group. In the series under investigation, the IC50 values ranged from 1.2 to approximately 30 nM against chloroquine-sensitive and multidrug-resistant Plasmodium falciparum strains. Modest to significant cross-resistance was noted in evaluation of these haloalkyl- and haloalkoxyquinolones for activity against the atovaquone-resistant clinical isolate Tm90-C2B, indicating that a primary target for some of these compounds is the parasite cytochrome bc1 complex. Additional evidence to support this biochemical mechanism includes the use of oxygen biosensor plate technology to show that the quinolone derivatives block oxygen consumption by parasitized red blood cells in a fashion similar to atovaquone in side-by-side experiments. Atovaquone is extremely potent and is the only drug in clinical use that targets the Plasmodium bc1 complex, but rapid emergence of resistance to it in both mono- and combination therapy is evident and therefore additional drugs are needed to target the cytochrome bc1 complex which are active against atovaquone-resistant parasites. Our study of a number of halogenated alkyl and alkoxy 4(1H)-quinolones highlights the potential for development of "endochin-like quinolones" (ELQ), bearing an extended trifluoroalkyl moiety at the 3-position, that exhibit selective antiplasmodial effects in the low nanomolar range and inhibitory activity against chloroquine and atovaquone-resistant parasites. Further studies of halogenated alkyl- and alkoxy-quinolones may lead to the development of safe and effective therapeutics for use in treatment or prevention of malaria and other parasitic diseases.
Figures


Similar articles
-
Endochin optimization: structure-activity and structure-property relationship studies of 3-substituted 2-methyl-4(1H)-quinolones with antimalarial activity.J Med Chem. 2010 Oct 14;53(19):7076-94. doi: 10.1021/jm1007903. J Med Chem. 2010. PMID: 20828199
-
Discovery, synthesis, and optimization of antimalarial 4(1H)-quinolone-3-diarylethers.J Med Chem. 2014 May 8;57(9):3818-34. doi: 10.1021/jm500147k. Epub 2014 Apr 18. J Med Chem. 2014. PMID: 24720377 Free PMC article.
-
Novel Endochin-Like Quinolones Exhibit Potent In Vitro Activity against Plasmodium knowlesi but Do Not Synergize with Proguanil.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02549-19. doi: 10.1128/AAC.02549-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32094134 Free PMC article.
-
Role of Trifluoromethyl Substitution in Design of Antimalarial Quinolones: a Comprehensive Review.Top Curr Chem (Cham). 2019 Mar 5;377(2):9. doi: 10.1007/s41061-019-0234-7. Top Curr Chem (Cham). 2019. PMID: 30835005 Review.
-
Hybrid molecules with potential in vitro antiplasmodial and in vivo antimalarial activity against drug-resistant Plasmodium falciparum.Med Res Rev. 2020 May;40(3):931-971. doi: 10.1002/med.21643. Epub 2019 Nov 6. Med Res Rev. 2020. PMID: 31692025 Review.
Cited by
-
Subtle changes in endochin-like quinolone structure alter the site of inhibition within the cytochrome bc1 complex of Plasmodium falciparum.Antimicrob Agents Chemother. 2015 Apr;59(4):1977-82. doi: 10.1128/AAC.04149-14. Epub 2015 Jan 20. Antimicrob Agents Chemother. 2015. PMID: 25605352 Free PMC article.
-
Identification and validation of tetracyclic benzothiazepines as Plasmodium falciparum cytochrome bc1 inhibitors.Chem Biol. 2011 Dec 23;18(12):1602-10. doi: 10.1016/j.chembiol.2011.09.016. Chem Biol. 2011. PMID: 22195562 Free PMC article.
-
Lead optimization of 3-carboxyl-4(1H)-quinolones to deliver orally bioavailable antimalarials.J Med Chem. 2012 May 10;55(9):4205-19. doi: 10.1021/jm201642z. Epub 2012 Apr 18. J Med Chem. 2012. PMID: 22435599 Free PMC article.
-
Plasmodium falciparum Mitochondrial Complex III, the Target of Atovaquone, Is Essential for Progression to the Transmissible Sexual Stages.Int J Mol Sci. 2024 Aug 26;25(17):9239. doi: 10.3390/ijms25179239. Int J Mol Sci. 2024. PMID: 39273187 Free PMC article.
-
New paradigms for understanding and step changes in treating active and chronic, persistent apicomplexan infections.Sci Rep. 2016 Jul 14;6:29179. doi: 10.1038/srep29179. Sci Rep. 2016. PMID: 27412848 Free PMC article.
References
-
- Ashley E, McGready R, Proux S, Nosten F. Malaria. Travel Medicine and Infectious Diseases. 2006;4:159–173. - PubMed
-
- Ashley EA, White NJ. Artemisinin-based combinations. Current Opinion in Infectious Diseases. 2005;18:531–536. - PubMed
-
- Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II) American Journal of Tropical Medicine and Hygiene. 2007;76:208–223. - PubMed
-
- Bray PG, Mungthin M, Hastings IM, Biagini GA, Saidu DK, Lakshmanan V, Johnson DJ, Hughes RH, Stocks PA, O'Neill PM, Fidock DA, Warhurst DC, Ward SA. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Molecular Microbiology. 2006;62:238–251. - PMC - PubMed
-
- Canfield CJ, Pudney M, Gutteridge WE. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Experimental Parasitology. 1995;80:373–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

