TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells
- PMID: 18082433
- PMCID: PMC2757006
- DOI: 10.1016/j.immuni.2007.11.011
TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells
Abstract
The T cell immunoglobulin mucin (TIM) proteins regulate T cell activation and tolerance. Here we showed that TIM-4 is expressed on human and mouse macrophages and dendritic cells, and both TIM-4 and TIM-1 specifically bound phosphatidylserine (PS) on the surface of apoptotic cells but not any other phospholipid tested. TIM-4(+) peritoneal macrophages, TIM-1(+) kidney cells, and TIM-4- or TIM-1-transfected cells efficiently phagocytosed apoptotic cells, and phagocytosis could be blocked by TIM-4 or TIM-1 monoclonal antibodies. Mutations in the unique cavity of TIM-4 eliminated PS binding and phagocytosis. TIM-4 mAbs that blocked PS binding and phagocytosis mapped to epitopes in this binding cavity. These results show that TIM-4 and TIM-1 are immunologically restricted members of the group of receptors whose recognition of PS is critical for the efficient clearance of apoptotic cells and prevention of autoimmunity.
Figures
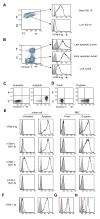

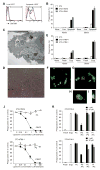
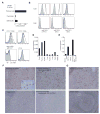

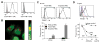
Comment in
-
Apoptotic PS to phagocyte TIM-4: eat me.Immunity. 2007 Dec;27(6):830-2. doi: 10.1016/j.immuni.2007.12.002. Immunity. 2007. PMID: 18093535
References
-
- Balasubramanian K, Chandra J, Schroit AJ. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J Biol Chem. 1997;272:31113–31117. - PubMed
-
- Beletskii A, Cooper M, Sriraman P, Chiriac C, Zhao L, Abbot S, Yu L. High-throughput phagocytosis assay utilizing a pH-sensitive fluorescent dye. Biotechniques. 2005;39:894–897. - PubMed
-
- Binne LL, Scott ML, Rennert PD. Human TIM-1 associates with the TCR complex and up-regulates T cell activation signals. J Immunol. 2007;178:4342–4350. - PubMed
-
- Borisenko GG, Matsura T, Liu SX, Tyurin VA, Jianfei J, Serinkan FB, Kagan VE. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells--existence of a threshold. Arch Biochem Biophys. 2003;413:41–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

