Lipopolysaccharide suppresses activation of the tuberomammillary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways
- PMID: 18082968
- PMCID: PMC2562932
- DOI: 10.1016/j.neuroscience.2007.10.042
Lipopolysaccharide suppresses activation of the tuberomammillary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways
Abstract
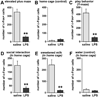
Infection and inflammation strongly inhibit a variety of behaviors, including exploration, social interaction, and food intake. The mechanisms that underlie sickness behavior remain elusive, but appear to involve fatigue and a state of hypo-arousal. Because histaminergic neurons in the ventral tuberomammillary nucleus of the hypothalamus (VTM) play a crucial role in the mediation of alertness and behavioral arousal, we investigated whether the histaminergic system represents a target for immune activation and, if so, whether modulation by ascending medullary immune-sensitive projections represents a possible mechanism. Rats were injected intraperitoneally with either the pro-inflammatory stimulus lipopolysaccharide (LPS) or saline, and exposed to one of various behavioral tests that would induce motivated behavior (exploration, play behavior, social interaction, sweetened milk consumption). Upon kill, brains were processed for c-Fos and histidine decarboxylase immunoreactivity. LPS treatment reduced behavioral activity and blocked behavioral test-associated c-Fos induction in histaminergic neurons of the VTM. These effects of LPS were prevented by prior inactivation of the caudal medullary dorsal vagal complex (DVC) with a local anesthetic. To determine whether LPS-responsive brainstem projection neurons might provide a link from the DVC to the VTM, the tracer Fluorogold was iontophoresed into the VTM a week prior to experiment. Retrogradely labeled neurons that expressed c-Fos in response to LPS treatment included catecholaminergic neurons within the nucleus of the solitary tract and ventrolateral medulla. These findings support the hypothesis that the histaminergic system represents an important component in the neurocircuitry relevant for sickness behavior that is linked to ascending pathways originating in the lower brainstem.
Figures








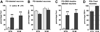


Similar articles
-
Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue?Brain Behav Immun. 2011 Mar;25(3):443-60. doi: 10.1016/j.bbi.2010.11.005. Epub 2010 Nov 12. Brain Behav Immun. 2011. PMID: 21075199 Free PMC article.
-
Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei.Brain Behav Immun. 2004 Mar;18(2):123-34. doi: 10.1016/j.bbi.2003.09.004. Brain Behav Immun. 2004. PMID: 14759590
-
Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior.Brain Behav Immun. 2009 Oct;23(7):926-30. doi: 10.1016/j.bbi.2009.03.005. Epub 2009 Mar 27. Brain Behav Immun. 2009. PMID: 19328847 Free PMC article.
-
Arousal and differential Fos expression in histaminergic neurons of the ascending arousal system during a feeding-related motivated behaviour.Eur J Neurosci. 2005 Apr;21(7):1931-42. doi: 10.1111/j.1460-9568.2005.04013.x. Eur J Neurosci. 2005. PMID: 15869486
-
[From biochemistry to pharmacology: the histaminergic neuron system in the brain].Nihon Yakurigaku Zasshi. 1992 Feb;99(2):63-81. doi: 10.1254/fpj.99.63. Nihon Yakurigaku Zasshi. 1992. PMID: 1559640 Review. Japanese.
Cited by
-
Suppression of Locomotor Activity in Female C57Bl/6J Mice Treated with Interleukin-1β: Investigating a Method for the Study of Fatigue in Laboratory Animals.PLoS One. 2015 Oct 15;10(10):e0140678. doi: 10.1371/journal.pone.0140678. eCollection 2015. PLoS One. 2015. PMID: 26469939 Free PMC article.
-
Impact of sanitary conditions and dietary amino acids on behaviour and brain monoamine levels in piglets.Brain Behav Immun Health. 2025 Aug 5;48:101076. doi: 10.1016/j.bbih.2025.101076. eCollection 2025 Oct. Brain Behav Immun Health. 2025. PMID: 40823320 Free PMC article.
-
How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry.Brain Behav Immun. 2008 Nov;22(8):1160-72. doi: 10.1016/j.bbi.2008.05.001. Epub 2008 Jun 17. Brain Behav Immun. 2008. PMID: 18562160 Free PMC article.
-
Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1.Front Neurosci. 2013 Jan 21;6:199. doi: 10.3389/fnins.2012.00199. eCollection 2012. Front Neurosci. 2013. PMID: 23346044 Free PMC article.
-
Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis.J Neurol Neurosurg Psychiatry. 2019 Jun;90(6):642-651. doi: 10.1136/jnnp-2018-320050. Epub 2019 Jan 25. J Neurol Neurosurg Psychiatry. 2019. PMID: 30683707 Free PMC article. Review.
References
-
- Aiche SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Com Neurol. 1996;373:62–75. - PubMed
-
- Becskei C, Riediger T, Hernadfalvy N, Arsenijevic D, Lutz TA, Langhans W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav Immun. 2007 epub ahead of print. - PubMed
-
- Buller K, Xu Y, Dayas C, Day T. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1β-induced hypothalamic pituitary adrenal axis responses. Neuroendocrinol. 2001;73:129–138. - PubMed
-
- Buller KM, Day TA. Systemic administration of interleukin-1β activates select populations of central amygdala afferents. J Comp Neurol. 2002;452:288–296. - PubMed
-
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

