Interactions of LSECtin and DC-SIGN/DC-SIGNR with viral ligands: Differential pH dependence, internalization and virion binding
- PMID: 18083206
- PMCID: PMC7103327
- DOI: 10.1016/j.virol.2007.11.001
Interactions of LSECtin and DC-SIGN/DC-SIGNR with viral ligands: Differential pH dependence, internalization and virion binding
Abstract
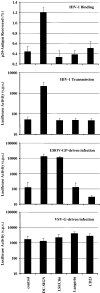
The calcium-dependent lectins DC-SIGN and DC-SIGNR (collectively termed DC-SIGN/R) bind to high-mannose carbohydrates on a variety of viruses. In contrast, the related lectin LSECtin does not recognize mannose-rich glycans and interacts with a more restricted spectrum of viruses. Here, we analyzed whether these lectins differ in their mode of ligand engagement. LSECtin and DC-SIGNR, which we found to be co-expressed by liver, lymph node and bone marrow sinusoidal endothelial cells, bound to soluble Ebola virus glycoprotein (EBOV-GP) with comparable affinities. Similarly, LSECtin, DC-SIGN and the Langerhans cell-specific lectin Langerin readily bound to soluble human immunodeficiency virus type-1 (HIV-1) GP. However, only DC-SIGN captured HIV-1 particles, indicating that binding to soluble GP is not necessarily predictive of binding to virion-associated GP. Capture of EBOV-GP by LSECtin triggered ligand internalization, suggesting that LSECtin like DC-SIGN might function as an antigen uptake receptor. However, the intracellular fate of lectin-ligand complexes might differ. Thus, exposure to low-pH medium, which mimics the acidic luminal environment in endosomes/lysosomes, released ligand bound to DC-SIGN/R but had no effect on LSECtin interactions with ligand. Our results reveal important differences between pathogen capture by DC-SIGN/R and LSECtin and hint towards different biological functions of these lectins.
Figures
References
-
- Appelmelk B.J., van D.I., Van Vliet S.J., Vandenbroucke-Grauls C.M., Geijtenbeek T.B., Van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003;170:1635–1639. - PubMed
-
- Baribaud F., Pöhlmann S., Sparwasser T., Kimata M.T., Choi Y.K., Haggarty B.S., Ahmad N., Macfarlan T., Edwards T.G., Leslie G.J., Arnason J., Reinhart T.A., Kimata J.T., Littman D.R., Hoxie J.A., Doms R.W. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 2001;75:10281–10289. - PMC - PubMed
-
- Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C., Van Vliet S.J., Eilering J.B., Martin M.P., Wu L., Martin T.D., Viebig N., Knolle P.A., KewalRamani V.N., Van Kooyk Y., Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001;193:671–678. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

