Interlaboratory performance of a microarray-based gene expression test to determine tissue of origin in poorly differentiated and undifferentiated cancers
- PMID: 18083688
- PMCID: PMC2175545
- DOI: 10.2353/jmoldx.2008.070099
Interlaboratory performance of a microarray-based gene expression test to determine tissue of origin in poorly differentiated and undifferentiated cancers
Abstract
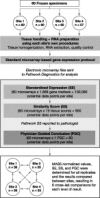
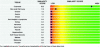
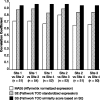
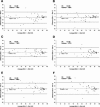
Clinical workup of metastatic malignancies of unknown origin is often arduous and expensive and is reported to be unsuccessful in 30 to 60% of cases. Accurate classification of uncertain primary cancers may improve with microarray-based gene expression testing. We evaluated the analytical performance characteristics of the Pathwork tissue of origin test, which uses expression signals from 1668 probe sets in a gene expression microarray, to quantify the similarity of tumor specimens to 15 known tissues of origin. Sixty archived tissue specimens from poorly and undifferentiated tumors (metastatic and primary) were analyzed at four laboratories representing a wide range of preanalytical conditions (eg, personnel, reagents, instrumentation, and protocols). Cross-laboratory comparisons showed highly reproducible results between laboratories, with correlation coefficients between 0.95 to 0.97 for measurements of similarity scores, and an average 93.8% overall concordance between laboratories in terms of final tissue calls. Bland-Altman plots (mean coefficients of reproducibility of 32.48+/-3.97) and kappa statistics (kappa >0.86) also indicated a high level of agreement between laboratories. We conclude that the Pathwork tissue of origin test is a robust assay that produces consistent results in diverse laboratory conditions reflecting the preanalytical variations found in the everyday clinical practice of molecular diagnostics laboratories.
Figures
References
-
- Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39:1990–2005. - PubMed
-
- Bugat R, Bataillard A, Lesimple T, Voigt JJ, Culine S, Lortholary A, Merrouche Y, Ganem G, Kaminsky MC, Negrier S, Perol M, Laforet C, Bedossa P, Bertrand G, Coindre JM, Fizazi K. FNCLCC: summary of the standards, options and recommendations for the management of patients with carcinoma of unknown primary site (2002) Br J Cancer. 2003;89:S59–S66. - PMC - PubMed
-
- DeYoung BR, Wick MR. Immunohistologic evaluation of metastatic carcinomas of unknown origin: an algorithmic approach. Semin Diagn Pathol. 2000;17:184–193. - PubMed
-
- Pavlidis N, Merrouche Y. The importance of identifying CUP subsets. In: Fizazi K, editor. Carcinoma of Unknown Primary Site. Taylor & Francis Group; New York: 2006. pp. 37–48.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

