Activation of cytosolic phospholipase A2alpha in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2
- PMID: 18083708
- PMCID: PMC4741307
- DOI: 10.1074/jbc.M709956200
Activation of cytosolic phospholipase A2alpha in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2
Abstract
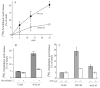
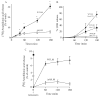
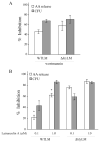
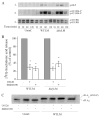
Eicosanoid production by macrophages is an early response to microbial infection that promotes acute inflammation. The intracellular pathogen Listeria monocytogenes stimulates arachidonic acid release and eicosanoid production from resident mouse peritoneal macrophages through activation of group IVA cytosolic phospholipase A2 (cPLA2alpha). The ability of wild type L. monocytogenes (WTLM) to stimulate arachidonic acid release is partially dependent on the virulence factor listeriolysin O; however, WTLM and L. monocytogenes lacking listeriolysin O (DeltahlyLM) induce similar levels of cyclooxygenase 2. Arachidonic acid release requires activation of MAPKs by WTLM and DeltahlyLM. The attenuated release of arachidonic acid that is observed in TLR2-/- and MyD88-/- macrophages infected with WTLM and DeltahlyLM correlates with diminished MAPK activation. WTLM but not DeltahlyLM increases intracellular calcium, which is implicated in regulation of cPLA2alpha. Prostaglandin E2, prostaglandin I2, and leukotriene C4 are produced by cPLA2alpha+/+ but not cPLA2alpha-/- macrophages in response to WTLM and DeltahlyLM. Tumor necrosis factor (TNF)-alpha production is significantly lower in cPLA2alpha+/+ than in cPLA2alpha-/- macrophages infected with WTLM and DeltahlyLM. Treatment of infected cPLA2alpha+/+ macrophages with the cyclooxygenase inhibitor indomethacin increases TNFalpha production to the level produced by cPLA2alpha-/- macrophages implicating prostaglandins in TNFalpha down-regulation. Therefore activation of cPLA2alpha in macrophages may impact immune responses to L. monocytogenes.
Figures


Similar articles
-
Prostaglandins from Cytosolic Phospholipase A2α/Cyclooxygenase-1 Pathway and Mitogen-activated Protein Kinases Regulate Gene Expression in Candida albicans-infected Macrophages.J Biol Chem. 2016 Mar 25;291(13):7070-86. doi: 10.1074/jbc.M116.714873. Epub 2016 Feb 3. J Biol Chem. 2016. PMID: 26841868 Free PMC article.
-
Low molecular weight hyaluronan activates cytosolic phospholipase A2α and eicosanoid production in monocytes and macrophages.J Biol Chem. 2014 Feb 14;289(7):4470-88. doi: 10.1074/jbc.M113.515106. Epub 2013 Dec 23. J Biol Chem. 2014. PMID: 24366870 Free PMC article. Clinical Trial.
-
Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans.J Biol Chem. 2010 Oct 1;285(40):30676-85. doi: 10.1074/jbc.M110.143800. Epub 2010 Jul 18. J Biol Chem. 2010. PMID: 20643646 Free PMC article.
-
Roles of factor increasing monocytopoiesis (FIM) and macrophage activation in host resistance to Listeria monocytogenes.Infection. 1988;16 Suppl 2:S137-40. doi: 10.1007/BF01639736. Infection. 1988. PMID: 3138186 Review.
-
Molecular aspects of Listeria monocytogenes infection.Pol J Microbiol. 2004;53 Suppl:17-22. Pol J Microbiol. 2004. PMID: 15787192 Review.
Cited by
-
Janus kinase 3 activity is necessary for phosphorylation of cytosolic phospholipase A2 and prostaglandin E2 synthesis by macrophages infected with Francisella tularensis live vaccine strain.Infect Immun. 2014 Mar;82(3):970-82. doi: 10.1128/IAI.01461-13. Epub 2013 Dec 16. Infect Immun. 2014. PMID: 24343645 Free PMC article.
-
The Contribution of Cytosolic Group IVA and Calcium-Independent Group VIA Phospholipase A2s to Adrenic Acid Mobilization in Murine Macrophages.Biomolecules. 2020 Apr 3;10(4):542. doi: 10.3390/biom10040542. Biomolecules. 2020. PMID: 32260121 Free PMC article.
-
Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy.Front Cell Infect Microbiol. 2014 May 12;4:51. doi: 10.3389/fcimb.2014.00051. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24860789 Free PMC article. Review.
-
Bacterial-Mediated Tumor Therapy: Old Treatment in a New Context.Adv Sci (Weinh). 2023 Apr;10(12):e2205641. doi: 10.1002/advs.202205641. Epub 2023 Mar 12. Adv Sci (Weinh). 2023. PMID: 36908053 Free PMC article. Review.
-
Cytosolic phospholipase A(2)α and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation.PLoS One. 2013 Jul 25;8(7):e69002. doi: 10.1371/journal.pone.0069002. eCollection 2013. PLoS One. 2013. PMID: 23950842 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

