The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds
- PMID: 18083798
- PMCID: PMC2245856
- DOI: 10.1104/pp.107.108183
The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds
Abstract
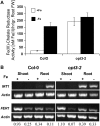
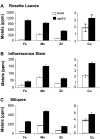
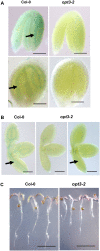
The Arabidopsis thaliana AtOPT3 belongs to the oligopeptide transporter (OPT) family, a relatively poorly characterized family of peptide/modified peptide transporters found in archebacteria, bacteria, fungi, and plants. A null mutation in AtOPT3 resulted in embryo lethality, indicating an essential role for AtOPT3 in embryo development. In this article, we report on the isolation and phenotypic characterization of a second AtOPT3 mutant line, opt3-2, harboring a T-DNA insertion in the 5' untranslated region of AtOPT3. The T-DNA insertion in the AtOPT3 promoter resulted in reduced but sufficient AtOPT3 expression to allow embryo formation in opt3-2 homozygous seeds. Phenotypic analyses of opt3-2 plants revealed three interesting loss-of-function phenotypes associated with iron metabolism. First, reduced AtOPT3 expression in opt3-2 plants resulted in the constitutive expression of root iron deficiency responses regardless of exogenous iron supply. Second, deregulation of root iron uptake processes in opt3-2 roots resulted in the accumulation of very high levels of iron in opt3-2 tissues. Hyperaccumulation of iron in opt3-2 resulted in the formation of brown necrotic areas in opt3-2 leaves and was more pronounced during the seed-filling stage. Third, reduced AtOPT3 expression resulted in decreased accumulation of iron in opt3-2 seeds. The reduced accumulation of iron in opt3-2 seeds is especially noteworthy considering the excessively high levels of accumulated iron in other opt3-2 tissues. AtOPT3, therefore, plays a critical role in two important aspects of iron metabolism, namely, maintenance of whole-plant iron homeostasis and iron nutrition of developing seeds.
Figures





References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281 32395–32402 - PubMed
-
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82 259–266 - PubMed
-
- Bellaoui M, Keddie JS, Gruissem W (2003) DCL is a plant-specific protein required for plastid ribosomal RNA processing and embryo development. Plant Mol Biol 53 531–543 - PubMed
-
- Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat AK (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J Biol Chem 275 13259–13265 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

