Escherichia coli cytosolic glycerophosphodiester phosphodiesterase (UgpQ) requires Mg2+, Co2+, or Mn2+ for its enzyme activity
- PMID: 18083802
- PMCID: PMC2238210
- DOI: 10.1128/JB.01223-07
Escherichia coli cytosolic glycerophosphodiester phosphodiesterase (UgpQ) requires Mg2+, Co2+, or Mn2+ for its enzyme activity
Abstract
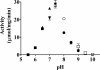
Escherichia coli cytosolic glycerophosphodiester phosphodiesterase, UgpQ, functions in the absence of other proteins encoded by the ugp operon and requires Mg2+, Mn2+, or Co2+, in contrast to Ca2+-dependent periplasmic glycerophosphodiester phosphodiesterase, GlpQ. UgpQ has broad substrate specificity toward various glycerophosphodiesters, producing sn-glycerol-3-phosphate and the corresponding alcohols. UgpQ accumulates under conditions of phosphate starvation, suggesting that it allows the utilization of glycerophosphodiesters as a source of phosphate. These results clarify how E. coli utilizes glycerophosphodiesters using two homologous enzymes, UgpQ and GlpQ.
Figures

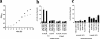


Similar articles
-
Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon.J Biol Chem. 1983 May 10;258(9):5428-32. J Biol Chem. 1983. PMID: 6304089
-
Purification and characterization of glpQ-encoded glycerophosphodiester phosphodiesterase from Escherichia coli K-12.Arch Biochem Biophys. 1988 Feb 1;260(2):577-84. doi: 10.1016/0003-9861(88)90484-5. Arch Biochem Biophys. 1988. PMID: 2829735
-
Characteristics of a ugp-encoded and phoB-dependent glycerophosphoryl diester phosphodiesterase which is physically dependent on the ugp transport system of Escherichia coli.J Bacteriol. 1988 Sep;170(9):4125-35. doi: 10.1128/jb.170.9.4125-4135.1988. J Bacteriol. 1988. PMID: 2842304 Free PMC article.
-
The ugp-encoded glycerophosphoryldiester phosphodiesterase, a transport-related enzyme of Escherichia coli.FEMS Microbiol Rev. 1989 Jun;5(1-2):115-24. doi: 10.1016/0168-6445(89)90015-6. FEMS Microbiol Rev. 1989. PMID: 2561262 Review. No abstract available.
-
Mammalian glycerophosphodiester phosphodiesterases.Biosci Biotechnol Biochem. 2007 Aug;71(8):1811-8. doi: 10.1271/bbb.70062. Epub 2007 Aug 7. Biosci Biotechnol Biochem. 2007. PMID: 17690467 Review.
Cited by
-
GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK.Science. 2013 Jan 18;339(6117):324-8. doi: 10.1126/science.1231921. Science. 2013. PMID: 23329048 Free PMC article.
-
Computational analysis of LexA regulons in Proteus species.3 Biotech. 2021 Mar;11(3):131. doi: 10.1007/s13205-021-02683-1. Epub 2021 Feb 19. 3 Biotech. 2021. PMID: 33680696 Free PMC article.
-
Rice and chickpea GDPDs are preferentially influenced by low phosphate and CaGDPD1 encodes an active glycerophosphodiester phosphodiesterase enzyme.Plant Cell Rep. 2016 Aug;35(8):1699-717. doi: 10.1007/s00299-016-1984-0. Epub 2016 Apr 23. Plant Cell Rep. 2016. PMID: 27108120
-
Escherichia coli adaptation and response to exposure to heavy atmospheric pollution.Sci Rep. 2019 Jul 26;9(1):10879. doi: 10.1038/s41598-019-47427-7. Sci Rep. 2019. PMID: 31350435 Free PMC article.
-
Comprehensive Genomics and Proteomics Analysis Reveals the Multiple Response Strategies of Endophytic Bacillus sp. WR13 to Iron Limitation.Microorganisms. 2023 Feb 1;11(2):367. doi: 10.3390/microorganisms11020367. Microorganisms. 2023. PMID: 36838332 Free PMC article.
References
-
- Bishop, R. E. 2005. The lipid A palmitoyltransferase PagP: molecular mechanisms and role in bacterial pathogenesis. Mol. Microbiol. 57900-912. - PubMed
-
- Brzoska, P., and W. Boos. 1989. The ugp-encoded glycerophosphoryldiester phosphodiesterase, a transport-related enzyme of Escherichia coli. FEMS Microbiol. Rev. 5115-124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

