Tn5 synaptic complex formation: role of transposase residue W450
- PMID: 18083803
- PMCID: PMC2238198
- DOI: 10.1128/JB.01488-07
Tn5 synaptic complex formation: role of transposase residue W450
Abstract
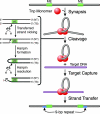
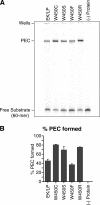
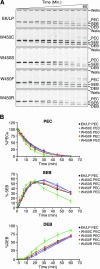
A series of Tn5 transposases (Tnp's) with mutations at the conserved amino acid position W450, which was structurally predicted to be important for synapsis, have been generated and characterized. This study demonstrates that W450 is involved in hydrophobic (and possibly aromatic) contacts within the Tnp monomer that negatively regulate synaptic complex formation.
Figures

Similar articles
-
Site-directed mutagenesis studies of tn5 transposase residues involved in synaptic complex formation.J Bacteriol. 2007 Oct;189(20):7436-41. doi: 10.1128/JB.00524-07. Epub 2007 Aug 10. J Bacteriol. 2007. PMID: 17693501 Free PMC article.
-
Tn5 transposase loops DNA in the absence of Tn5 transposon end sequences.Mol Microbiol. 2006 Dec;62(6):1558-68. doi: 10.1111/j.1365-2958.2006.05471.x. Mol Microbiol. 2006. PMID: 17074070
-
Mutation of Tn5 transposase beta-loop residues affects all steps of Tn5 transposition: the role of conformational changes in Tn5 transposition.Biochemistry. 2006 Dec 26;45(51):15552-62. doi: 10.1021/bi061227v. Epub 2006 Dec 5. Biochemistry. 2006. PMID: 17176076 Free PMC article.
-
Tn5 as a model for understanding DNA transposition.Mol Microbiol. 2003 Mar;47(5):1199-206. doi: 10.1046/j.1365-2958.2003.03382.x. Mol Microbiol. 2003. PMID: 12603728 Review.
-
Structure/function insights into Tn5 transposition.Curr Opin Struct Biol. 2004 Feb;14(1):50-7. doi: 10.1016/j.sbi.2004.01.008. Curr Opin Struct Biol. 2004. PMID: 15102449 Review.
References
-
- Betermier, M., R. Alazard, F. Ragueh, E. Roulet, A. Toussaint, and M. Chandler. 1987. Phage Mu transposase: deletion of the carboxy-terminal end does not abolish DNA-binding activity. Mol. Gen. Genet. 21077-85. - PubMed
-
- Bhasin, A., I. Y. Goryshin, M. Steiniger-White, D. York, and W. S. Reznikoff. 2000. Characterization of a Tn5 pre-cleavage synaptic complex. J. Mol. Biol. 30249-63. - PubMed
-
- Davies, D. R., I. Y. Goryshin, W. S. Reznikoff, and I. Rayment. 2000. Three-dimensional structure of the Tn5 synaptic complex transposition intermediate. Science 28977-85. - PubMed
-
- Davies, D. R., L. Mahnke Braam, W. S. Reznikoff, and I. Rayment. 1999. The three-dimensional structure of a Tn5 transposase-related protein determined to 2.9-Å resolution. J. Biol. Chem. 27411904-11913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

