Impact of ammonium permeases mepA, mepB, and mepC on nitrogen-regulated secondary metabolism in Fusarium fujikuroi
- PMID: 18083831
- PMCID: PMC2238153
- DOI: 10.1128/EC.00351-07
Impact of ammonium permeases mepA, mepB, and mepC on nitrogen-regulated secondary metabolism in Fusarium fujikuroi
Abstract
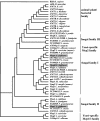
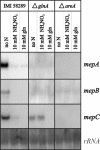
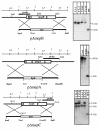
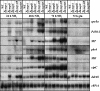
In Fusarium fujikuroi, the production of gibberellins and bikaverin is repressed by nitrogen sources such as glutamine or ammonium. Sensing and uptake of ammonium by specific permeases play key roles in nitrogen metabolism. Here, we describe the cloning of three ammonium permease genes, mepA, mepB, and mepC, and their participation in ammonium uptake and signal transduction in F. fujikuroi. The expression of all three genes is strictly regulated by the nitrogen regulator AreA. Severe growth defects of DeltamepB mutants on low-ammonium medium and methylamine uptake studies suggest that MepB functions as the main ammonium permease in F. fujikuroi. In DeltamepB mutants, nitrogen-regulated genes such as the gibberellin and bikaverin biosynthetic genes are derepressed in spite of high extracellular ammonium concentrations. mepA mepB and mepC mepB double mutants show a similar phenotype as DeltamepB mutants. All three F. fujikuroi mep genes fully complemented the Saccharomyces cerevisiae mep1 mep2 mep3 triple mutant to restore growth on low-ammonium medium, whereas only MepA and MepC restored pseudohyphal growth in the mep2/mep2 mutant. Overexpression of mepC in the DeltamepB mutants partially suppressed the growth defect but did not prevent derepression of AreA-regulated genes. These studies provide evidence that MepB functions as a regulatory element in a nitrogen sensing system in F. fujikuroi yet does not provide the sensor activity of Mep2 in yeast, indicating differences in the mechanisms by which nitrogen is sensed in S. cerevisiae and F. fujikuroi.
Figures







Similar articles
-
The General Amino Acid Permease FfGap1 of Fusarium fujikuroi Is Sorted to the Vacuole in a Nitrogen-Dependent, but Npr1 Kinase-Independent Manner.PLoS One. 2015 Apr 24;10(4):e0125487. doi: 10.1371/journal.pone.0125487. eCollection 2015. PLoS One. 2015. PMID: 25909858 Free PMC article.
-
Recent advances in metabolic regulation and bioengineering of gibberellic acid biosynthesis in Fusarium fujikuroi.World J Microbiol Biotechnol. 2022 Jun 11;38(8):131. doi: 10.1007/s11274-022-03324-2. World J Microbiol Biotechnol. 2022. PMID: 35689127 Review.
-
The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae.EMBO J. 1998 Aug 10;17(5):1236-47. doi: 10.1093/emboj/17.5.1236. EMBO J. 1998. PMID: 9482721 Free PMC article.
-
Nitrogen repression of deoxynivalenol biosynthesis is mediated by Mep2 ammonium permease in Fusarium graminearum.Environ Microbiol. 2022 Nov;24(11):5392-5407. doi: 10.1111/1462-2920.16233. Epub 2022 Oct 18. Environ Microbiol. 2022. PMID: 36200537
-
Secondary metabolism in Fusarium fujikuroi: strategies to unravel the function of biosynthetic pathways.Appl Microbiol Biotechnol. 2018 Jan;102(2):615-630. doi: 10.1007/s00253-017-8679-5. Epub 2017 Dec 4. Appl Microbiol Biotechnol. 2018. PMID: 29204899 Review.
Cited by
-
The General Amino Acid Permease FfGap1 of Fusarium fujikuroi Is Sorted to the Vacuole in a Nitrogen-Dependent, but Npr1 Kinase-Independent Manner.PLoS One. 2015 Apr 24;10(4):e0125487. doi: 10.1371/journal.pone.0125487. eCollection 2015. PLoS One. 2015. PMID: 25909858 Free PMC article.
-
Light-dependent functions of the Fusarium fujikuroi CryD DASH cryptochrome in development and secondary metabolism.Appl Environ Microbiol. 2013 Apr;79(8):2777-88. doi: 10.1128/AEM.03110-12. Epub 2013 Feb 15. Appl Environ Microbiol. 2013. PMID: 23417004 Free PMC article.
-
Recent advances in metabolic regulation and bioengineering of gibberellic acid biosynthesis in Fusarium fujikuroi.World J Microbiol Biotechnol. 2022 Jun 11;38(8):131. doi: 10.1007/s11274-022-03324-2. World J Microbiol Biotechnol. 2022. PMID: 35689127 Review.
-
The AreA transcription factor mediates the regulation of deoxynivalenol (DON) synthesis by ammonium and cyclic adenosine monophosphate (cAMP) signalling in Fusarium graminearum.Mol Plant Pathol. 2015 Dec;16(9):987-99. doi: 10.1111/mpp.12254. Epub 2015 May 4. Mol Plant Pathol. 2015. PMID: 25781642 Free PMC article.
-
Metarhizium robertsii ammonium permeases (MepC and Mep2) contribute to rhizoplane colonization and modulates the transfer of insect derived nitrogen to plants.PLoS One. 2019 Oct 16;14(10):e0223718. doi: 10.1371/journal.pone.0223718. eCollection 2019. PLoS One. 2019. PMID: 31618269 Free PMC article.
References
-
- Biswas, K., and J. Morschhäuser. 2005. The Mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Mol. Microbiol. 56649-669. - PubMed
-
- Boeckstaens, M., B. Andre, and A. M. Marini. 2007. The yeast ammonium transport protein Mep2 and its positive regulator, the Npr1 kinase, play an important role in normal and pseudohyphal growth on various nitrogen media through retrieval of excreted ammonium. Mol. Microbiol. 64534-546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

