Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells
- PMID: 18083846
- PMCID: PMC2275001
- DOI: 10.1182/blood-2007-08-110114
Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells
Abstract
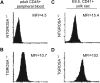
Definitive hematopoietic stem and progenitor cells (HSCs/Ps) originating from the yolk sac and/or para-aorta-splanchno-pleura/aorta-gonad-mesonephros are hypothesized to colonize the fetal liver, but mechanisms involved are poorly defined. The Rac subfamily of Rho GTPases has been shown to play essential roles in HSC/P localization to the bone marrow following transplantation. Here, we study the role of Rac1 in HSC/P migration during ontogeny and seeding of fetal liver. Using a triple-transgenic approach, we have deleted Rac1 in HSCs/Ps during very early embryonic development. Without Rac1, there was a decrease in circulating HSCs/Ps in the blood of embryonic day (E) 10.5 embryos, while yolk sac definitive hematopoiesis was quantitatively normal. Intraembryonic hematopoiesis was significantly impaired in Rac1-deficient embryos, culminating with absence of intra-aortic clusters and fetal liver hematopoiesis. At E10.5, Rac1-deficient HSCs/Ps displayed decreased transwell migration and impaired inter-action with the microenvironment in migration-dependent assays. These data suggest that Rac1 plays an important role in HSC/P migration during embryonic development and is essential for the emergence of intraembryonic hematopoiesis.
Figures



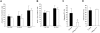
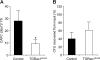
References
-
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. - PubMed
-
- Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Hematol. 1970;18:279–296. - PubMed
-
- Weissman I, Papaioannou V, Gardner R. Fetal hematopoietic origins of the adult hematolymphoid system. In: Clarkson B, Marks PA, Till JE, editors. Differentiation of Normal and Neoplastic Hematopoietic Cells. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1978. pp. 371–387.
-
- Johnson GR, Moore MA. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975;258:726–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

