Suppressing posttranslational gluconoylation of heterologous proteins by metabolic engineering of Escherichia coli
- PMID: 18083862
- PMCID: PMC2258596
- DOI: 10.1128/AEM.01790-07
Suppressing posttranslational gluconoylation of heterologous proteins by metabolic engineering of Escherichia coli
Abstract
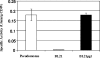
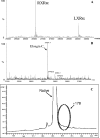
Minimization of chemical modifications during the production of proteins for pharmaceutical and medical applications is of fundamental and practical importance. The gluconoylation of heterologously expressed protein which is observed in Escherichia coli BL21(DE3) constitutes one such undesired posttranslational modification. We postulated that formation of gluconoylated/phosphogluconoylated products of heterologous proteins is caused by the accumulation of 6-phosphogluconolactone due to the absence of phosphogluconolactonase (PGL) in the pentose phosphate pathway. The results obtained demonstrate that overexpression of a heterologous PGL in BL21(DE3) suppresses the formation of the gluconoylated adducts in the therapeutic proteins studied. When this E. coli strain was grown in high-cell-density fed-batch cultures with an extra copy of the pgl gene, we found that the biomass yield and specific productivity of a heterologous 18-kDa protein increased simultaneously by 50 and 60%, respectively. The higher level of PGL expression allowed E. coli strain BL21(DE3) to satisfy the extra demand for precursors, as well as the energy requirements, in order to replicate plasmid DNA and express heterologous genes, as metabolic flux analysis showed by the higher precursor and NADPH fluxes through the oxidative branch of the pentose phosphate shunt. This work shows that E. coli strain BL21(DE3) can be used as a host to produce three different proteins, a heterodimer of liver X receptors, elongin C, and an 18-kDa protein. This is the first report describing a novel and general strategy for suppressing this nonenzymatic modification by metabolic pathway engineering.
Figures

Similar articles
-
6-Phosphogluconolactonase is critical for the efficient functioning of the pentose phosphate pathway.FEBS J. 2024 Oct;291(20):4459-4472. doi: 10.1111/febs.17221. Epub 2024 Jul 10. FEBS J. 2024. PMID: 38982839
-
Proteomic profiling of Escherichia coli proteins under high cell density fed-batch cultivation with overexpression of phosphogluconolactonase.Biotechnol Prog. 2005 Sep-Oct;21(5):1401-11. doi: 10.1021/bp050048m. Biotechnol Prog. 2005. PMID: 16209543
-
High-yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum in Escherichia coli.Microb Cell Fact. 2016 Aug 12;15(1):141. doi: 10.1186/s12934-016-0536-1. Microb Cell Fact. 2016. PMID: 27520031 Free PMC article.
-
Escherichia coli ORF ybhE is pgl gene encoding 6-phosphogluconolactonase (EC 3.1.1.31) that has no homology with known 6PGLs from other organisms.FEMS Microbiol Lett. 2005 Mar 15;244(2):275-80. doi: 10.1016/j.femsle.2005.01.050. FEMS Microbiol Lett. 2005. PMID: 15766779
-
Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels.J Mol Biol. 1996 Jul 19;260(3):289-98. doi: 10.1006/jmbi.1996.0399. J Mol Biol. 1996. PMID: 8757792 Review.
Cited by
-
Improvements in large-scale production of tobacco etch virus protease.Protein Expr Purif. 2025 Apr;228:106648. doi: 10.1016/j.pep.2024.106648. Epub 2024 Dec 15. Protein Expr Purif. 2025. PMID: 39681152
-
Metabolite damage and its repair or pre-emption.Nat Chem Biol. 2013 Feb;9(2):72-80. doi: 10.1038/nchembio.1141. Nat Chem Biol. 2013. PMID: 23334546 Review.
-
Reduction of N-terminal methionylation while increasing titer by lowering metabolic and protein production rates in E. coli auto-induced fed-batch fermentation.J Ind Microbiol Biotechnol. 2012 Aug;39(8):1199-208. doi: 10.1007/s10295-012-1127-8. Epub 2012 Apr 20. J Ind Microbiol Biotechnol. 2012. PMID: 22526331
-
The NMR signature of gluconoylation: a frequent N-terminal modification of isotope-labeled proteins.J Biomol NMR. 2019 Feb;73(1-2):71-79. doi: 10.1007/s10858-019-00228-6. Epub 2019 Feb 8. J Biomol NMR. 2019. PMID: 30737614 Free PMC article.
-
Cloning, functional characterization and genomic organization of 1,8-cineole synthases from Lavandula.Plant Mol Biol. 2012 Jul;79(4-5):393-411. doi: 10.1007/s11103-012-9920-3. Epub 2012 May 17. Plant Mol Biol. 2012. PMID: 22592779
References
-
- Baneyx, F. 1999. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 10:411-421. - PubMed
-
- Beranek, M., J. Drsata, and V. Palicka. 2001. Inhibitory effect of glycation on catalytic activity of alanine aminotransferase. Mol. Cell. Biochem. 218:35-39. - PubMed
-
- Berti, P. J., I. Ekiel, P. Lindahl, M. Abrahamson, and A. C. Storer. 1997. Affinity purification and elimination of methionine oxidation in recombinant human cystatin C. Protein Expr. Purif. 11:111-118. - PubMed
-
- Casey, E. B., H. R. Zhao, and E. C. Abraham. 1995. Role of glycine1 and lysine2 in the glycation of bovine gamma-B-crystallin. J. Biol. Chem. 270:20781-20786. - PubMed
-
- Cortassa, S., J. C. Aon, and M. A. Aon. 1995. Fluxes of carbon, phosphorylation, and redox intermediates during growth of Saccharomyces cerevisiae on different carbon sources. Biotechnol. Bioeng. 47:193-208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

