Effect of pH on intracellular accumulation of trace concentrations of Hg(II) in Escherichia coli under anaerobic conditions, as measured using a mer-lux bioreporter
- PMID: 18083863
- PMCID: PMC2227699
- DOI: 10.1128/AEM.00717-07
Effect of pH on intracellular accumulation of trace concentrations of Hg(II) in Escherichia coli under anaerobic conditions, as measured using a mer-lux bioreporter
Abstract
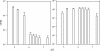
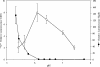
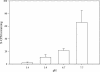
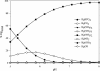
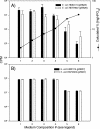
The effects of pH on the uptake and accumulation of Hg(II) by Escherichia coli were determined at trace, environmentally relevant, concentrations of Hg and under anaerobic conditions. Hg(II) accumulation was measured using inducible light production from E. coli HMS174 harboring a mer-lux bioreporter plasmid (pRB28). The effect of pH on the toxicity of higher concentrations of Hg(II) was measured using a constitutive lux plasmid (pRB27) in the same bacterial host. In this study, intracellular accumulation and toxicity of Hg(II) under anaerobic conditions were both significantly enhanced with decreasing pH over the pH range of 8 to 5. The pH effect on Hg(II) accumulation was most pronounced at pHs of <6, which substantially enhanced the Hg(II)-dependent light response. This enhanced response did not appear to be due to pH stress, as similar results were obtained whether cells were grown at the same pH as the assay or at a different pH. The enhanced accumulation of Hg(II) was also not related to differences in the chemical speciation of Hg(II) in the external medium resulting from the changes in pH. Experiments with Cd(II), also detectable by the mer-lux bioreporter system, showed that Cd(II) accumulation responded differently to pH changes than the net accumulation of Hg(II). Potential implications of these findings for our understanding of bacterial accumulation of Hg(II) under anaerobic conditions and for bacteria-mediated cycling of Hg(II) in aquatic ecosystems are discussed. Arguments are provided suggesting that this differential accumulation is due to changes in uptake of mercury.
Figures


References
-
- Barkay, T., R. R. Turner, L. D. Rasmussen, C. A. Kelly, and J. W. M. Rudd. 1998. Luminescence facilitated detection of bioavailable mercury in natural waters, p. 231-246. In R. A. Rossa (ed.), Bioluminescence methods and protocols. Humana Press, Totowa, NJ. - PubMed
-
- Barkay, T., S. M. Miller, and A. O. Summers. 2003. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27:355-384. - PubMed
-
- Beard, S. J., R. Hashim, G. Wu, M. R. B. Binet, M. N. Hughes, and R. K. Poole. 2000. Evidence for the transport of zinc(II) ions via the Pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol. Lett. 184:231-235. - PubMed
-
- Benoit, J. M., C. C. Gilmour, and R. P. Mason. 2001. Aspects of bioavailability of mercury for methylation in pure cultures of Desulfobulbus propionicus (1pr3). Environ. Sci. Technol. 35:127-132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

