The type II secretion system of Legionella pneumophila elaborates two aminopeptidases, as well as a metalloprotease that contributes to differential infection among protozoan hosts
- PMID: 18083880
- PMCID: PMC2227731
- DOI: 10.1128/AEM.01944-07
The type II secretion system of Legionella pneumophila elaborates two aminopeptidases, as well as a metalloprotease that contributes to differential infection among protozoan hosts
Abstract
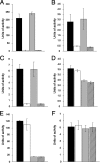
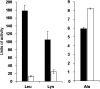
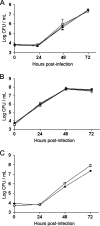
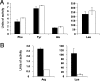
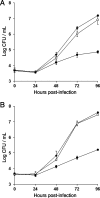
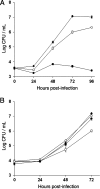
Legionella pneumophila, the agent of Legionnaires' disease, is an intracellular parasite of aquatic amoebae and human macrophages. A key factor for L. pneumophila in intracellular infection is its type II protein secretion system (Lsp). In order to more completely define Lsp output, we recently performed a proteomic analysis of culture supernatants. Based upon the predictions of that analysis, we found that L. pneumophila secretes two distinct aminopeptidase activities encoded by the genes lapA and lapB. Whereas lapA conferred activity against leucine, phenylalanine, and tyrosine aminopeptides, lapB was linked to the cleavage of lysine- and arginine-containing substrates. To assess the role of secreted aminopeptidases in intracellular infection, we examined the relative abilities of lapA and lapB mutants to infect human U937 cell macrophages as well as Hartmannella vermiformis and Acanthamoeba castellanii amoebae. Although these experiments identified a dispensable role for LapA and LapB, they uncovered a previously unrecognized role for the type II-dependent ProA (MspA) metalloprotease. Whereas proA mutants were not defective for macrophage or A. castellanii infection, they (but not their complemented derivatives) were impaired for growth upon coculture with H. vermiformis. Thus, ProA represents the first type II effector implicated in an intracellular infection event. Furthermore, proA represents an L. pneumophila gene that shows differential importance among protozoan infection models, suggesting that the legionellae might have evolved some of its factors to especially target certain of their protozoan hosts.
Figures
Similar articles
-
Type II Secretion-Dependent Aminopeptidase LapA and Acyltransferase PlaC Are Redundant for Nutrient Acquisition during Legionella pneumophila Intracellular Infection of Amoebas.mBio. 2018 Apr 17;9(2):e00528-18. doi: 10.1128/mBio.00528-18. mBio. 2018. PMID: 29666285 Free PMC article.
-
Multiple Legionella pneumophila Type II secretion substrates, including a novel protein, contribute to differential infection of the amoebae Acanthamoeba castellanii, Hartmannella vermiformis, and Naegleria lovaniensis.Infect Immun. 2013 May;81(5):1399-410. doi: 10.1128/IAI.00045-13. Epub 2013 Feb 19. Infect Immun. 2013. PMID: 23429532 Free PMC article.
-
A type II secreted RNase of Legionella pneumophila facilitates optimal intracellular infection of Hartmannella vermiformis.Microbiology (Reading). 2009 Mar;155(Pt 3):882-890. doi: 10.1099/mic.0.023218-0. Microbiology (Reading). 2009. PMID: 19246759 Free PMC article.
-
Pathogenicity of Legionella pneumophila.Int J Med Microbiol. 2001 Nov;291(5):331-43. doi: 10.1078/1438-4221-00139. Int J Med Microbiol. 2001. PMID: 11727817 Review.
-
Legionella pneumophila adaptation to intracellular life and the host response: clues from genomics and transcriptomics.FEBS Lett. 2007 Jun 19;581(15):2829-38. doi: 10.1016/j.febslet.2007.05.026. Epub 2007 May 21. FEBS Lett. 2007. PMID: 17531986 Review.
Cited by
-
The major facilitator superfamily-type protein LbtC promotes the utilization of the legiobactin siderophore by Legionella pneumophila.Microbiology (Reading). 2012 Mar;158(Pt 3):721-735. doi: 10.1099/mic.0.055533-0. Epub 2011 Dec 8. Microbiology (Reading). 2012. PMID: 22160401 Free PMC article.
-
Type II Secretion Is Necessary for Optimal Association of the Legionella-Containing Vacuole with Macrophage Rab1B but Enhances Intracellular Replication Mainly by Rab1B-Independent Mechanisms.Infect Immun. 2016 Nov 18;84(12):3313-3327. doi: 10.1128/IAI.00750-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27600508 Free PMC article.
-
Type II Secretion Substrates of Legionella pneumophila Translocate Out of the Pathogen-Occupied Vacuole via a Semipermeable Membrane.mBio. 2017 Jun 20;8(3):e00870-17. doi: 10.1128/mBio.00870-17. mBio. 2017. PMID: 28634242 Free PMC article.
-
Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila.Future Microbiol. 2009 Sep;4(7):797-805. doi: 10.2217/fmb.09.53. Future Microbiol. 2009. PMID: 19722835 Free PMC article. Review.
-
Amoebae as training grounds for microbial pathogens.mBio. 2024 Aug 14;15(8):e0082724. doi: 10.1128/mbio.00827-24. Epub 2024 Jul 8. mBio. 2024. PMID: 38975782 Free PMC article. Review.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Aragon, V., O. Rossier, and N. P. Cianciotto. 2002. Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223-2231. - PubMed
-
- Arima, J., Y. Uesugi, M. Uraji, S. Yatsushiro, S. Tsuboi, M. Iwabuchi, and T. Hatanaka. 2006. Modulation of Streptomyces leucine aminopeptidase by calcium: identification and functional analysis of key residues in activation and stabilization by calcium. J. Biol. Chem. 281:5885-5894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

