Use of a recombinant fluorescent substrate with cleavage sites for all botulinum neurotoxins in high-throughput screening of natural product extracts for inhibitors of serotypes A, B, and E
- PMID: 18083881
- PMCID: PMC2227718
- DOI: 10.1128/AEM.01690-07
Use of a recombinant fluorescent substrate with cleavage sites for all botulinum neurotoxins in high-throughput screening of natural product extracts for inhibitors of serotypes A, B, and E
Abstract
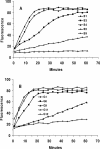
The seven serotypes of botulinum neurotoxin (BoNTs) are zinc metalloproteases that cleave and inactivate proteins critical for neurotransmission. The synaptosomal protein of 25 kDa (SNAP-25) is cleaved by BoNTs A, C, and E, while vesicle-associated membrane protein (VAMP) is the substrate for BoNTs B, D, F, and G. BoNTs not only are medically useful drugs but also are potential bioterrorist and biowarfare threat agents. Because BoNT protease activity is required for toxicity, inhibitors of that activity might be effective for antibotulinum therapy. To expedite inhibitor discovery, we constructed a hybrid gene encoding (from the N terminus to the C terminus, with respect to the expressed product) green fluorescent protein, then a SNAP-25 fragment encompassing residues Met-127 to Gly-206, and then VAMP residues Met-1 to Lys-94. Cysteine was added as the C terminus. The expressed product, which contained the protease cleavage sites for all seven botulinum serotypes, was purified and coupled covalently through the C-terminal sulfhydryl group to maleimide-activated 96-well plates. The substrate was readily cleaved by BoNTs A, B, D, E, and F. Using this assay and an automated 96-well pipettor, we screened 528 natural product extracts for inhibitors of BoNT A, B, and E protease activities. Serotype-specific inhibition was found in 30 extracts, while 5 others inhibited two serotypes.
Figures
Similar articles
-
Development of a fusion protein SNVP as substrate for assaying multi-serotype botulinum neurotoxins.Anal Biochem. 2014 Oct 15;463:75-81. doi: 10.1016/j.ab.2013.06.019. Epub 2013 Jul 12. Anal Biochem. 2014. PMID: 23851341
-
Structure of botulinum neurotoxin type D light chain at 1.65 A resolution: repercussions for VAMP-2 substrate specificity.Biochemistry. 2006 Mar 14;45(10):3255-62. doi: 10.1021/bi052518r. Biochemistry. 2006. PMID: 16519520
-
Botulinum neurotoxin serotype F: identification of substrate recognition requirements and development of inhibitors with low nanomolar affinity.Biochemistry. 2005 Mar 15;44(10):4067-73. doi: 10.1021/bi0477642. Biochemistry. 2005. PMID: 15751983
-
Fluorigenic substrates for the protease activities of botulinum neurotoxins, serotypes A, B, and F.Appl Environ Microbiol. 2003 Jan;69(1):297-303. doi: 10.1128/AEM.69.1.297-303.2003. Appl Environ Microbiol. 2003. PMID: 12514008 Free PMC article.
-
Optimization of SNAP-25 and VAMP-2 Cleavage by Botulinum Neurotoxin Serotypes A-F Employing Taguchi Design-of-Experiments.Toxins (Basel). 2019 Oct 11;11(10):588. doi: 10.3390/toxins11100588. Toxins (Basel). 2019. PMID: 31614566 Free PMC article.
Cited by
-
A cross-over inhibitor of the botulinum neurotoxin light chain B: a natural product implicating an exosite mechanism of action.Chem Commun (Camb). 2011 Feb 14;47(6):1713-5. doi: 10.1039/c0cc04078a. Epub 2011 Jan 4. Chem Commun (Camb). 2011. PMID: 21203627 Free PMC article.
-
Tyrosine phosphorylation of botulinum neurotoxin protease domains.Front Pharmacol. 2012 Jun 4;3:102. doi: 10.3389/fphar.2012.00102. eCollection 2012. Front Pharmacol. 2012. PMID: 22675300 Free PMC article.
-
Isolation and quantification of botulinum neurotoxin from complex matrices using the BoTest matrix assays.J Vis Exp. 2014 Mar 3;(85):51170. doi: 10.3791/51170. J Vis Exp. 2014. PMID: 24638074 Free PMC article.
-
Multi-wavelength Spatial LED illumination based detector for in vitro detection of Botulinum Neurotoxin A Activity.Sens Actuators B Chem. 2010 Apr 8;146(1-8):297-306. doi: 10.1016/j.snb.2010.02.009. Sens Actuators B Chem. 2010. PMID: 20498728 Free PMC article.
-
Detection of botulinum neurotoxin serotype A, B, and F proteolytic activity in complex matrices with picomolar to femtomolar sensitivity.Appl Environ Microbiol. 2012 Nov;78(21):7687-97. doi: 10.1128/AEM.01664-12. Epub 2012 Aug 24. Appl Environ Microbiol. 2012. PMID: 22923410 Free PMC article.
References
-
- Ahmed, S. A., and L. A. Smith. 2000. Light chain of botulinum A neurotoxin expressed as an inclusion body from a synthetic gene is catalytically and functionally active. J. Protein Chem. 19:475-487. - PubMed
-
- Anne, C., F. Cornille, C. Lenoir, and B. P. Roques. 2001. High-throughput fluorigenic assay for determination of botulinum type B neurotoxin protease activity. Anal. Biochem. 291:253-261. - PubMed
-
- Ansiaux, R., C. Baudelet, G. O. Cron, J. Segers, C. Dessy, P. Martinive, J. De Wever, J. Verrax, V. Wauthier, N. Beghein, V. Gregoire, P. Buc Calderon, O. Feron, and B. Gallez. 2006. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin. Cancer Res. 12:1276-1283. - PubMed
-
- Arnon, S. S., R. Schechter, T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, J. Hauer, M. Layton, S. Lillibridge, M. T. Osterholm, T. O'Toole, G. Parker, P. K. Russel, D. L. Swerdlow, and K. Tonat. 2001. Botulinum toxin as a biological weapon. JAMA 285:1059-1070. - PubMed
-
- Bade, S., A. Rummel, C. Reisinger, T. Karnath, G. Ahnert-Hilger, H. Bigalke, and T. J. Binz. 2004. Botulinum neurotoxin type D enables cytosolic delivery of enzymatically active cargo proteins to neurones via unfolded translocation intermediates. J. Neurochem. 91:1461-1472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

