Axin1 and Axin2 are regulated by TGF- and mediate cross-talk between TGF- and Wnt signaling pathways
- PMID: 18083923
- PMCID: PMC2647987
- DOI: 10.1196/annals.1402.082
Axin1 and Axin2 are regulated by TGF- and mediate cross-talk between TGF- and Wnt signaling pathways
Abstract
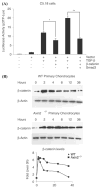
Chondrocyte maturation during endochondral bone formation is regulated by a number of signals that either promote or inhibit maturation. Among these, two well-studied signaling pathways play crucial roles in modulating chondrocyte maturation: transforming growth factor-beta (TGF-beta)/Smad3 signaling slows the rate of chondrocyte maturation, while Wingless/INT-1-related (Wnt)/beta-catenin signaling enhances the rate of chondrocyte maturation. Axin1 and Axin2 are functionally equivalent and have been shown to inhibit Wnt/beta-catenin signaling and stimulate TGF-beta signaling. Here we show that while Wnt3a stimulates Axin2 in a negative feedback loop, TGF-beta suppresses the expression of both Axin1 and Axin2 and stimulates beta-catenin signaling. In Axin2 -/- chondrocytes, TGF-beta treatment results in a sustained increase in beta-catenin levels compared to wild-type chondrocytes. In contrast, overexpression of Axin enhanced TGF-beta signaling while overexpression of beta-catenin inhibited the ability of TGF-beta to induce Smad3-sensitive reporters. Finally, the suppression of the Axins is Smad3-dependent since the effect is absent in Smad3 -/- chondrocytes. Altogether these findings show that the Axins act to integrate signals between the Wnt/beta-catenin and TGF-beta/Smad pathways. Since the suppression Axin1 and Axin2 expression by TGF-beta reduces TGF-beta signaling and enhances Wnt/beta-catenin signaling, the overall effect is a shift from TGF-beta toward Wnt/beta-catenin signaling and an acceleration of chondrocyte maturation.
Figures

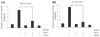





References
-
- Ferguson CM, et al. Smad 2 and 3 mediate TGF-β1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. - PubMed
-
- Iwamoto M, et al. Runx2 expression and action in chondrocytes are regulated by retinoid signaling and parathyroid hormone-related peptide (PTHrP) Osteoarthr Cart. 2003;11:6–15. - PubMed
-
- Ionescu AM, et al. PTHrP modulates chondrocyte differentiation through AP-1 and CREB signaling. J Biol Chem. 2001;276:11639–11647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

