Analysis of intermolecular base pair formation of prohead RNA of the phage phi29 DNA packaging motor using NMR spectroscopy
- PMID: 18084020
- PMCID: PMC2241910
- DOI: 10.1093/nar/gkm874
Analysis of intermolecular base pair formation of prohead RNA of the phage phi29 DNA packaging motor using NMR spectroscopy
Abstract
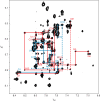
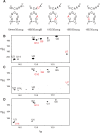
The bacteriophage ø29 DNA packaging motor that assembles on the precursor capsid (prohead) contains an essential 174-nt structural RNA (pRNA) that forms multimers. To determine the structural features of the CE- and D-loops believed to be involved in multimerization of pRNA, 35- and 19-nt RNA molecules containing the CE-loop or the D-loop, respectively, were produced and shown to form a heterodimer in a Mg2+-dependent manner, similar to that with full-length pRNA. It has been hypothesized that four intermolecular base pairs are formed between pRNA molecules. Our NMR study of the heterodimer, for the first time, proved directly the existence of two intermolecular Watson-Crick G-C base pairs. The two potential intermolecular A-U base pairs were not observed. In addition, flexibility of the D-loop was found to be important since a Watson-Crick base pair introduced at the base of the D-loop disrupted the formation of the intermolecular G-C hydrogen bonds, and therefore affected heterodimerization. Introduction of this mutation into the biologically active 120-nt pRNA (U80C mutant) resulted in no detectable dimerization at ambient temperature as shown by native gel and sedimentation velocity analyses. Interestingly, this pRNA bound to prohead and packaged DNA as well as the wild-type 120-nt pRNA.
Figures






References
-
- Grimes S, Jardine PJ, Anderson D. Bacteriophage phi 29 DNA packaging. Adv. Virus Res. 2002;58:255–294. - PubMed
-
- Guo PX, Erickson S, Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNA. Science. 1987;236:690–694. - PubMed
-
- Zhang F, Lemieux S, Wu X, St-Arnaud D, McMurray CT, Major F, Anderson D. Function of hexameric RNA in packaging of bacteriophage phi 29 DNA in vitro. Mol. Cell. 1998;2:141–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

