A thermosensory pathway that controls body temperature
- PMID: 18084288
- PMCID: PMC2423341
- DOI: 10.1038/nn2027
A thermosensory pathway that controls body temperature
Abstract
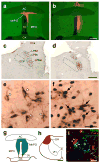
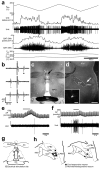
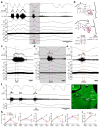
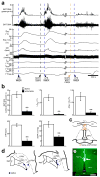
Defending body temperature against environmental thermal challenges is one of the most fundamental homeostatic functions that are governed by the nervous system. Here we describe a somatosensory pathway that essentially constitutes the afferent arm of the thermoregulatory reflex that is triggered by cutaneous sensation of environmental temperature changes. Using in vivo electrophysiological and anatomical approaches in the rat, we found that lateral parabrachial neurons are pivotal in this pathway by glutamatergically transmitting cutaneous thermosensory signals received from spinal somatosensory neurons directly to the thermoregulatory command center, the preoptic area. This feedforward pathway mediates not only sympathetic and shivering thermogenic responses but also metabolic and cardiac responses to skin cooling challenges. Notably, this 'thermoregulatory afferent' pathway exists in parallel with the spinothalamocortical somatosensory pathway that mediates temperature perception. These findings make an important contribution to our understanding of both the somatosensory system and thermal homeostasis -- two mechanisms that are fundamental to the nervous system and to our survival.
Figures

References
-
- Hammel HT. Regulation of internal body temperature. Annu Rev Physiol. 1968;30:641–710. - PubMed
-
- Hensel H. Thermoreception and temperature regulation. Monographs of the Physiological Society No. 38. Academic press; London: 1981. - PubMed
-
- Boulant JA, Gonzalez RR. The effect of skin temperature on the hypothalamic control of heat loss and heat production. Brain Res. 1977;120:367–372. - PubMed
-
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

