MuSK controls where motor axons grow and form synapses
- PMID: 18084289
- PMCID: PMC2923649
- DOI: 10.1038/nn2026
MuSK controls where motor axons grow and form synapses
Erratum in
- Nat Neurosci. 2008 Feb;11(2):238
Abstract
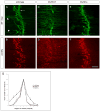
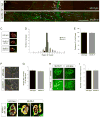
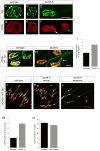
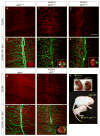
Motor axons approach muscles that are regionally prespecialized, as acetylcholine receptors are clustered in the central region of muscle before and independently of innervation. This muscle prepattern requires MuSK, a receptor tyrosine kinase that is essential for synapse formation. It is not known how muscle prepatterning is established, and whether motor axons recognize this prepattern. Here we show that expression of Musk is prepatterned in muscle and that early Musk expression in developing myotubes is sufficient to establish muscle prepatterning. We further show that ectopic Musk expression promotes ectopic synapse formation, indicating that muscle prepatterning normally has an instructive role in directing where synapses will form. In addition, ectopic Musk expression stimulates synapse formation in the absence of Agrin and rescues the lethality of Agrn mutant mice, demonstrating that the postsynaptic cell, and MuSK in particular, has a potent role in regulating the formation of synapses.
Conflict of interest statement
The authors do not have a conflict of interest related to this work.
Figures



References
-
- Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Curr Opin Neurobiol. 2002;12:100–103. - PubMed
-
- Bennett MR, Pettigrew AG. The formation of neuromuscular synapses. Cold Spring Harb Symp Quant Biol. 1976;40:409–424. - PubMed
-
- Brennan KJ, Hardeman EC. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J Biol Chem. 1993;268:719–725. - PubMed
-
- Burden SJ. The formation of neuromuscular synapses. Genes Dev. 1998;12:133–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

