Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions
- PMID: 18084303
- PMCID: PMC2922956
- DOI: 10.1038/nsmb1350
Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions
Abstract
The heterophilic synaptic adhesion molecules neuroligins and neurexins are essential for establishing and maintaining neuronal circuits by modulating the formation and maturation of synapses. The neuroligin-neurexin adhesion is Ca2+-dependent and regulated by alternative splicing. We report a structure of the complex at a resolution of 2.4 A between the mouse neuroligin-1 (NL1) cholinesterase-like domain and the mouse neurexin-1beta (NX1beta) LNS (laminin, neurexin and sex hormone-binding globulin-like) domain. The structure revealed a delicate neuroligin-neurexin assembly mediated by a hydrophilic, Ca2+-mediated and solvent-supplemented interface, rendering it capable of being modulated by alternative splicing and other regulatory factors. Thermodynamic data supported a mechanism wherein splicing site B of NL1 acts by modulating a salt bridge at the edge of the NL1-NX1beta interface. Mapping neuroligin mutations implicated in autism indicated that most such mutations are structurally destabilizing, supporting deficient neuroligin biosynthesis and processing as a common cause for this brain disorder.
Figures

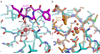

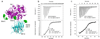
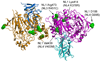
References
-
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. - PubMed
-
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

