Synergistic depression of NMDA receptor-mediated transmission by ketamine, ketoprofen and L-NAME combinations in neonatal rat spinal cords in vitro
- PMID: 18084314
- PMCID: PMC2267281
- DOI: 10.1038/sj.bjp.0707638
Synergistic depression of NMDA receptor-mediated transmission by ketamine, ketoprofen and L-NAME combinations in neonatal rat spinal cords in vitro
Abstract
Background and purpose: Spinal N-methyl-D-aspartate (NMDA) receptor/cyclooxygenase (COX) and nitric oxide synthase (NOS) pathways play a major role in nociceptive processing, and influencing them simultaneously may induce synergistic analgesia. This study determined the spinal antinociceptive interactions between ketamine (NMDA receptor channel blocker), ketoprofen (COX inhibitor) and L-NAME (NOS inhibitor) combinations.
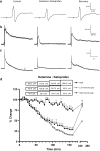
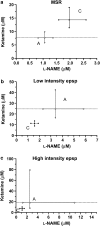
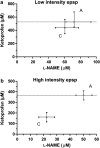
Experimental approach: Using an in vitro neonatal rat spinal cord preparation, two A-fibre-mediated reflexes, the monosynaptic reflex (MSR) and the low-intensity excitatory postsynaptic potential (epsp), and one C-fibre-mediated reflex, the high-intensity epsp, were evoked electrically. The effect of drugs and drug combinations on these reflexes was assessed and the type of interaction determined by isobolographic analysis.
Key results: Infusion of ketamine alone decreased all three reflexes. That of ketoprofen decreased both the low and the high-intensity epsp only. Infusion of L-NAME alone produced no significant effects. Co-infusion of fixed ratios of IC(40) fractions of both (ketamine+ketoprofen) and (ketamine+L-NAME) were synergistic for depressing the low and the high-intensity epsps. The interaction was sub-additive for both combinations on the MSR. The only significant effect for the (ketoprofen+L-NAME) combination was synergism on the high-intensity epsp.
Conclusions and implications: All three combinations synergistically depressed nociceptive spinal transmission, and both ketamine and ketoprofen and ketamine and L-NAME combinations did so with potentially decreased motor side effects. If such combination profiles also occur in vivo, the present findings raise the possibility of ultimate therapeutic exploitation of increased analgesia with fewer side effects.
Figures


References
-
- Ashina M, Lassen LH, Bendtsen L, Jensen R, Olesen J. Effect of inhibition of nitric oxide synthase on chronic tension-type headache: a randomised crossover trial. Lancet. 1999;353:287–289. - PubMed
-
- Ates M, Hamza M, Seidel K, Kotalla CE, Ledent C, Gühring H. Intrathecally applied flurbiprofen produces an endocannabinoid-dependent antinociception in the rat formalin test. Eur J Neurosci. 2003;17:597–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

