Endothelial alpha1-adrenoceptors regulate neo-angiogenesis
- PMID: 18084315
- PMCID: PMC2267280
- DOI: 10.1038/sj.bjp.0707637
Endothelial alpha1-adrenoceptors regulate neo-angiogenesis
Abstract
Background and purpose: Intact endothelium plays a pivotal role in post-ischaemic angiogenesis. It is a phenomenon finely tuned by activation and inhibition of several endothelial receptors. The presence of alpha(1)-adrenoceptors on the endothelium suggests that these receptors may participate in regenerative phenomena by regulating the responses of endothelial cells involved in neo-angiogenesis.
Experimental approach: We evaluated the expression of the subtypes of the alpha(1)-adrenoceptor in isolated endothelial cells harvested from Wistar-Kyoto (WKY) rats. We explored the possibility these alpha(1)-adrenoceptors may influence the pro-angiogenic phenotype of endothelial cells in vitro. In vivo, we used a model of hindlimb ischaemia in WKY rats, to assess the effects of alpha(1) adrenoceptor agonist or antagonist on angiogenesis in the ischaemic hindlimb by laser Doppler blood flow measurements, digital angiographies, hindlimb perfusion with dyed beads and histological evaluation.
Key results: In vitro, pharmacological antagonism of alpha(1)-adrenoceptors in endothelial cells from WKY rats by doxazosin enhanced, while stimulation of these adrenoceptors with phenylephrine, inhibited endothelial cell proliferation and DNA synthesis, ERK and retinoblastoma protein (Rb) phosphorylation, cell migration and tubule formation. In vivo, we found increased alpha(1)-adrenoceptor density in the ischaemic hindlimb, compared to non-ischaemic hindlimb, suggesting an enhanced alpha(1)-adrenoceptor tone in the ischaemic tissue. Treatment with doxazosin (0.06 mg kg(-1) day(-1) for 14 days) did not alter systemic blood pressure but enhanced neo-angiogenesis in the ischaemic hindlimb, as measured by all our assays.
Conclusions: Our findings support the hypothesis that the alpha(1)-adrenoceptors in endothelial cells provide a negative regulation of angiogenesis.
Figures





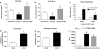

References
-
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor–Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. - PubMed
-
- Alexandrov A, Keffel S, Goepel M, Michel MC. Stimulation of alpha1A-adrenoceptors in rat-1 cells inhibits extracellular signal-regulated kinase by activating p38 mitogen-activated protein kinase. Mol Pharmacol. 1998;54:755–760. - PubMed
-
- Augustin HG. Tubes, branches, and pillars: the many ways of forming a new vasculature. Circ Res. 2001;89:645–647. - PubMed
-
- Ben-Dov IZ, Ben-Arie L, Mekler J, Bursztyn M. How should patients treated with alpha-blockers be followed? Insights from an ambulatory blood pressure monitoring database. J Hypertens. 2006;24:861–865. - PubMed
-
- Benning CM, Kyprianou N. Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Res. 2002;62:597–602. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

