Inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation on tumor-associated endothelial cells leads to treatment of orthotopic human colon cancer in nude mice
- PMID: 18084614
- PMCID: PMC2134903
- DOI: 10.1593/neo.07667
Inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation on tumor-associated endothelial cells leads to treatment of orthotopic human colon cancer in nude mice
Abstract
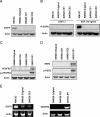
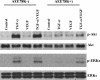
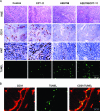
The purpose of our study was to determine whether the dual inhibition of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) signaling pathways in tumor-associated endothelial cells can inhibit the progressive growth of human colon carcinoma in the cecum of nude mice. SW620CE2 human colon cancer cells growing in culture and orthotopically in the cecum of nude mice expressed a high level of transforming growth factor alpha (TGF-alpha) and vascular endothelial growth factor (VEGF) but were negative for EGFR, human epidermal growth factor receptor 2 (HER2), and VEGFR. Double immunofluorescence staining revealed that tumor-associated endothelial cells expressed EGFR, VEGFR2, phosphorylated EGFR (pEGFR), and phosphorylated VEGFR (pVEGFR). Treatment of mice with either 7H-pyrrolo [2,3-d]-pyrimidine lead scaffold (AEE788; an inhibitor of EGFR and VEGFR tyrosine kinase) or CPT-11 as single agents significantly inhibited the growth of cecal tumors (P < .01); this decrease was even more pronounced with AEE788 combined with CPT-11 (P < .001). AEE788 alone or combined with CPT-11 also inhibited the expression of pEGFR and pVEGFR on tumor-associated endothelial cells, significantly decreased vascularization and tumor cell proliferation, and increased the level of apoptosis in both tumor-associated endothelial cells and tumor cells. These data demonstrate that targeting EGFR and VEGFR signaling on tumor-associated endothelial cells provides a viable approach for the treatment of colon cancer.
Keywords: AEE788; Endothelial cells; colon cancer; dual inhibition; tyrosine kinase receptors.
Figures



References
-
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. - PubMed
-
- Bond JH. Colorectal cancer update: prevention, screening, treatment, and surveillance for high-risk groups. Med Clin North Am. 2000;84:1163–1182. - PubMed
-
- Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. (Review) - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. (Timeline) - PubMed
-
- Stoeltzing O, Liu W, Reinmuth N, Parikh A, Ahmad SA, Jung YD, Fan F, Ellis LM. Angiogenesis and antiangiogenic therapy of colon cancer liver metastasis. Ann Surg Oncol. 2003;10:722–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
