Statins stimulate in vitro membrane FasL expression and lymphocyte apoptosis through RhoA/ROCK pathway in murine melanoma cells
- PMID: 18084615
- PMCID: PMC2134904
- DOI: 10.1593/neo.07727
Statins stimulate in vitro membrane FasL expression and lymphocyte apoptosis through RhoA/ROCK pathway in murine melanoma cells
Abstract
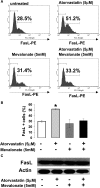
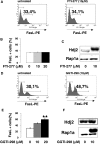
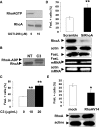
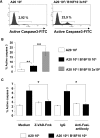
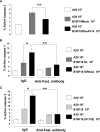
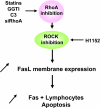
The capacity of FasL molecules expressed on melanoma cells to induce lymphocyte apoptosis contributes to either antitumor immune response or escape depending on their expression level. Little is known, however, about the mechanisms regulating FasL protein expression. Using the murine B16F10 melanoma model weakly positive for FasL, we demonstrated that in vitro treatment with statins, inhibitors of 3-hydroxy-3-methylgutaryl CoA reductase, enhances membrane FasL expression. C3 exotoxin and the geranylgeranyl transferase I inhibitor GGTI-298, but not the farnesyl transferase inhibitor FTI-277, mimic this effect. The capacity of GGTI-298 and C3 exotoxin to inhibit RhoA activity prompted us to investigate the implication of RhoA in FasL expression. Inhibition of RhoA expression by small interfering RNA (siRNA) increased membrane FasL expression, whereas overexpression of constitutively active RhoA following transfection of RhoAV14 plasmid decreased it. Moreover, the inhibition of a RhoA downstream effector p160ROCK also induced this FasL overexpression. We conclude that the RhoA/ROCK pathway negatively regulates membrane FasL expression in these melanoma cells. Furthermore, we have shown that B16F10 cells, through the RhoA/ROCK pathway, promote in vitro apoptosis of Fas-sensitive A20 lymphoma cells. Our results suggest that RhoA/ROCK inhibition could be an interesting target to control FasL expression and lymphocyte apoptosis induced by melanoma cells.
Keywords: FasL; Melanoma; RhoA; apoptosis; statins.
Figures

Similar articles
-
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors decrease Fas ligand expression and cytotoxicity in activated human T lymphocytes.Circulation. 2003 Sep 23;108(12):1506-13. doi: 10.1161/01.CIR.0000089086.48617.2B. Epub 2003 Sep 2. Circulation. 2003. PMID: 12952848
-
3-Hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors, atorvastatin and simvastatin, induce apoptosis of vascular smooth muscle cells by downregulation of Bcl-2 expression and Rho A prenylation.Atherosclerosis. 2002 Mar;161(1):17-26. doi: 10.1016/s0021-9150(01)00613-x. Atherosclerosis. 2002. PMID: 11882313
-
Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux.J Biol Chem. 2005 Jun 10;280(23):22212-21. doi: 10.1074/jbc.M502761200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15817453
-
On the role and significance of Fas (Apo-1/CD95) ligand (FasL) expression in immune privileged tissues and cancer cells using multiple myeloma as a model.Leuk Lymphoma. 1998 Nov;31(5-6):477-90. doi: 10.3109/10428199809057607. Leuk Lymphoma. 1998. PMID: 9922038 Review.
-
Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease.Pharmacol Ther. 2014 Jul;143(1):87-110. doi: 10.1016/j.pharmthera.2014.02.007. Epub 2014 Feb 26. Pharmacol Ther. 2014. PMID: 24582968 Free PMC article. Review.
Cited by
-
Autocrine secretion of 15d-PGJ2 mediates simvastatin-induced apoptotic burst in human metastatic melanoma cells.Br J Pharmacol. 2014 Dec;171(24):5708-27. doi: 10.1111/bph.12871. Br J Pharmacol. 2014. PMID: 25091578 Free PMC article.
-
Autocrine amplification loop in statin-induced apoptosis of human melanoma cells.Br J Pharmacol. 2009 Aug;157(7):1278-90. doi: 10.1111/j.1476-5381.2009.00298.x. Epub 2009 Jun 25. Br J Pharmacol. 2009. PMID: 19563533 Free PMC article.
-
Lipid traits and lipid-lowering drug target genes and risk of melanoma: a mendelian randomization study.Arch Dermatol Res. 2024 May 31;316(6):301. doi: 10.1007/s00403-024-03100-2. Arch Dermatol Res. 2024. PMID: 38819656
-
Repositioning of HMG-CoA Reductase Inhibitors as Adjuvants in the Modulation of Efflux Pump-Mediated Bacterial and Tumor Resistance.Antibiotics (Basel). 2023 Sep 20;12(9):1468. doi: 10.3390/antibiotics12091468. Antibiotics (Basel). 2023. PMID: 37760764 Free PMC article. Review.
-
Rho kinase proteins--pleiotropic modulators of cell survival and apoptosis.Anticancer Res. 2011 Nov;31(11):3645-57. Anticancer Res. 2011. PMID: 22110183 Free PMC article. Review.
References
-
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. - PubMed
-
- Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. - PubMed
-
- Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. - PubMed
-
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
