Hypoxia and the presence of human vascular endothelial cells affect prostate cancer cell invasion and metabolism
- PMID: 18084621
- PMCID: PMC2134910
- DOI: 10.1593/neo.07568
Hypoxia and the presence of human vascular endothelial cells affect prostate cancer cell invasion and metabolism
Abstract
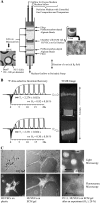
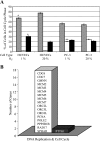
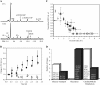
Tumor progression and metastasis are influenced by hypoxia, as well as by interactions between cancer cells and components of the stroma, such as endothelial cells. Here, we have used a magnetic resonance (MR)-compatible invasion assay to further understand the effects of hypoxia on human prostate cancer cell invasion and metabolism in the presence and absence of human umbilical vein endothelial cells (HUVECs). Additionally, we compared endogenous activities of selected proteases related to invasion in PC-3 cells and HUVECs, profiled gene expression of PC-3 cells by microarray, and evaluated cell proliferation of PC-3 cells and HUVECs by flow cytometry, under hypoxic and oxygenated conditions. The invasion of less-invasive DU-145 cells was not affected by either hypoxia or the presence of HUVECs. However, hypoxia significantly decreased the invasion of PC-3 cells. This hypoxia-induced decrease was attenuated by the presence of HUVECs, whereas under oxygenated conditions, HUVECs did not alter the invasion of PC-3 cells. Cell metabolism changed distinctly with hypoxia and invasion. The endogenous activity of selected extracellular proteases, although altered by hypoxia, did not fully explain the hypoxia-induced changes in invasion. Gene expression profiling indicated that hypoxia affects multiple cellular functions and pathways.
Keywords: Prostate cancer; endothelial/cancer cell interaction; hypoxia; invasion; magnetic resonance (MR) imaging and spectroscopy.
Figures






Similar articles
-
Lymphatic endothelial cells actively regulate prostate cancer cell invasion.NMR Biomed. 2016 Jul;29(7):904-11. doi: 10.1002/nbm.3543. Epub 2016 May 5. NMR Biomed. 2016. PMID: 27149683
-
Noninvasive MRI of endothelial cell response to human breast cancer cells.Neoplasia. 2006 Mar;8(3):207-13. doi: 10.1593/neo.05547. Neoplasia. 2006. PMID: 16611414 Free PMC article.
-
Elevated cell invasion is induced by hypoxia in a human pituitary adenoma cell line.Cell Adh Migr. 2007 Jan-Mar;1(1):43-51. Epub 2007 Jan 27. Cell Adh Migr. 2007. PMID: 19262092 Free PMC article.
-
IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction.Prostate. 2015 Jun;75(8):883-95. doi: 10.1002/pros.22971. Epub 2015 Feb 14. Prostate. 2015. PMID: 25683512 Free PMC article.
-
Rhapontigenin inhibited hypoxia inducible factor 1 alpha accumulation and angiogenesis in hypoxic PC-3 prostate cancer cells.Biol Pharm Bull. 2011;34(6):850-5. doi: 10.1248/bpb.34.850. Biol Pharm Bull. 2011. PMID: 21628883
Cited by
-
Radial Flow Perfusion Enables Real-Time Profiling of Cellular Metabolism at Low Oxygen Levels with Hyperpolarized 13C NMR Spectroscopy.Metabolites. 2021 Aug 26;11(9):576. doi: 10.3390/metabo11090576. Metabolites. 2021. PMID: 34564392 Free PMC article.
-
MRI of metastasis-permissive microenvironments.Future Oncol. 2011 Nov;7(11):1269-84. doi: 10.2217/fon.11.114. Future Oncol. 2011. PMID: 22044202 Free PMC article. Review.
-
Enzymatically Regulated Peptide Pairing and Catalysis for the Bioanalysis of Extracellular Prometastatic Activities of Functionally Linked Enzymes.Sci Rep. 2016 May 3;6:25362. doi: 10.1038/srep25362. Sci Rep. 2016. PMID: 27140831 Free PMC article.
-
Neoplasia: the second decade.Neoplasia. 2008 Dec;10(12):1314-24. doi: 10.1593/neo.81372. Neoplasia. 2008. PMID: 19048110 Free PMC article.
-
Hypoxia triggers major metabolic changes in AML cells without altering indomethacin-induced TCA cycle deregulation.ACS Chem Biol. 2011 Feb 18;6(2):169-75. doi: 10.1021/cb900300j. Epub 2010 Oct 18. ACS Chem Biol. 2011. PMID: 20886892 Free PMC article.
References
-
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. - PubMed
-
- Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3:659–671. - PubMed
-
- Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
