Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells
- PMID: 18084640
- PMCID: PMC2139984
- DOI: 10.1017/S1740925X07000579
Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells
Abstract
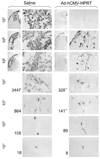
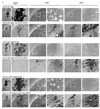
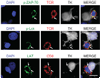
First-generation adenovirus can be engineered with powerful promoters to drive expression of therapeutic transgenes. Numerous clinical trials for glioblastoma multiforme using first generation adenoviral vectors have either been performed or are ongoing, including an ongoing, Phase III, multicenter trial in Europe and Israel (Ark Therapeutics, Inc.). Although in the absence of anti-adenovirus immune responses expression in the brain lasts 6-18 months, systemic infection with adenovirus induces immune responses that inhibit dramatically therapeutic transgene expression from first generation adenoviral vectors, thus, potentially compromising therapeutic efficacy. Here, we show evidence of an immunization threshold for the dose that generates an immune response strong enough to eliminate transgene expression from the CNS. For the systemic immunization to eliminate transgene expression from the brain, > or = 1 x 10(7) infectious units (iu) of adenovirus need to be used as immunogen. Furthermore, this immune response eliminates >90% of transgene expression from 1 x 10(7)-1 x 10(3) iu of vector injected into the striatum 60 days earlier. Importantly, elimination of transgene expression is independent of the nature of the promoter that drives transgene expression and is accompanied by brain infiltration of CD8(+) T cells and macrophages. In conclusion, once the threshold for systemic immunization (i.e. 1 x 10(7) iu) is crossed, the immune response eliminates transgene expression by >90% even from brains that receive as little as 1000 iu of adenoviral vectors, independently of the type of promoter that drives expression.
Keywords: Gene therapy; adenovirus; neuroimmunology; promoter; threshold.
Figures



Similar articles
-
Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions.Curr Gene Ther. 2007 Oct;7(5):347-60. doi: 10.2174/156652307782151498. Curr Gene Ther. 2007. PMID: 17979681 Free PMC article. Review.
-
Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral-induced brain inflammation, but delays immune system-mediated elimination of transgene expression.Mol Ther. 2003 Sep;8(3):400-11. doi: 10.1016/s1525-0016(03)00178-3. Mol Ther. 2003. PMID: 12946313 Free PMC article.
-
Immune regulation of transgene expression in the brain: B cells regulate an early phase of elimination of transgene expression from adenoviral vectors.Viral Immunol. 2006 Summer;19(3):508-17. doi: 10.1089/vim.2006.19.508. Viral Immunol. 2006. PMID: 16987068 Free PMC article.
-
Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses.Hum Gene Ther. 2005 Jun;16(6):741-51. doi: 10.1089/hum.2005.16.741. Hum Gene Ther. 2005. PMID: 15960605 Free PMC article.
-
Immunology of neurological gene therapy: how T cells modulate viral vector-mediated therapeutic transgene expression through immunological synapses.Neurotherapeutics. 2007 Oct;4(4):715-24. doi: 10.1016/j.nurt.2007.07.010. Neurotherapeutics. 2007. PMID: 17920552 Free PMC article. Review.
Cited by
-
Exogenous fms-like tyrosine kinase 3 ligand overrides brain immune privilege and facilitates recognition of a neo-antigen without causing autoimmune neuropathology.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14443-8. doi: 10.1073/pnas.0913496107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660723 Free PMC article.
-
Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: implications for a glioma phase 1 clinical trial.Hum Gene Ther Methods. 2012 Aug;23(4):271-84. doi: 10.1089/hgtb.2012.060. Epub 2012 Sep 5. Hum Gene Ther Methods. 2012. PMID: 22950971 Free PMC article.
-
Clinical and Translational Landscape of Viral Gene Therapies.Cells. 2024 Nov 19;13(22):1916. doi: 10.3390/cells13221916. Cells. 2024. PMID: 39594663 Free PMC article. Review.
-
Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions.Curr Gene Ther. 2007 Oct;7(5):347-60. doi: 10.2174/156652307782151498. Curr Gene Ther. 2007. PMID: 17979681 Free PMC article. Review.
-
Immune-mediated loss of transgene expression from virally transduced brain cells is irreversible, mediated by IFNγ, perforin, and TNFα, and due to the elimination of transduced cells.Mol Ther. 2012 Apr;20(4):808-19. doi: 10.1038/mt.2011.243. Epub 2012 Jan 10. Mol Ther. 2012. PMID: 22233583 Free PMC article.
References
-
- Akrigg A, Wilkinson GW, Oram JD. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Research. 1985;2:107–121. - PubMed
-
- Amalfitano A, Parks RJ. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Current Gene Therapy. 2002;2:111–133. - PubMed
-
- Bemelmans AP, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Human Gene Therapy. 1999;10:2987–2997. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

