Adipose Tissue: A Metabolic Regulator. Potential Implications for the Metabolic Outcome of Subjects Born Small for Gestational Age (SGA)
- PMID: 18084671
- PMCID: PMC2174062
- DOI: 10.1900/RDS.2007.4.134
Adipose Tissue: A Metabolic Regulator. Potential Implications for the Metabolic Outcome of Subjects Born Small for Gestational Age (SGA)
Abstract
Adipose tissue is involved in the regulation of glucose and lipid metabolism, energy balance, inflammation and immune response. Abdominal obesity plays a key role in the development of insulin resistance because of the high lipolytic rate of visceral adipose tissue and its secretion of adipocytokines. Low birth weight subjects are prone to central redistribution of adipose tissue and are at high risk of developing metabolic syndrome, type 2 diabetes and cardiovascular disease. Intrauterine adipogenesis may play a key role in the fetal origin of the pathogenesis of metabolic syndrome, type 2 diabetes and cardiovascular disease. Therefore, knowledge of the behavior of visceral adipose tissue-derived stem cells could provide a greater understanding of the metabolic risk related to intrauterine growth retardation, with potential clinical implications for the prevention of long-term metabolic alterations.
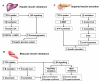
Figures




References
-
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. - PubMed
-
- Saltiel AR. You are what you secrete. Nat Med. 2001;7:887–888. - PubMed
-
- Vettor R, Milan G, Rossato M, Federspil G. Review article: adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;22(Suppl 2):3–10. - PubMed
-
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The third National Health and Nutrition Examination Survey 1988-1994. Diabetes Care. 1998;21:518–524. - PubMed
-
- Pi-Sunyer F. Weight and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1996;63(Suppl 3):426S–429S. - PubMed
LinkOut - more resources
Full Text Sources
