Expression Analysis of cPLA2 Alpha Interacting TIP60 in Diabetic KKAy and Non-Diabetic C57BL Wild-Type Mice: No Impact of Transient and Stable TIP60 Overexpression on Glucose-Stimulated Insulin Secretion in Pancreatic Beta-Cells
- PMID: 18084672
- PMCID: PMC2174065
- DOI: 10.1900/RDS.2007.4.147
Expression Analysis of cPLA2 Alpha Interacting TIP60 in Diabetic KKAy and Non-Diabetic C57BL Wild-Type Mice: No Impact of Transient and Stable TIP60 Overexpression on Glucose-Stimulated Insulin Secretion in Pancreatic Beta-Cells
Abstract
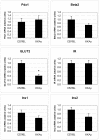
In the present study we investigate the expression levels of cytosolic phospholipase A2 alpha (cPLA2alpha) interacting histone acetyl transferase proteins TIP60alpha and TIP60beta in non-diabetic C57BL wild-type mice and obese type 2 diabetic KKAy model mice. The aim was to test our hypothesis that TIP60 plays a regulatory role in glucose-stimulated insulin secretion from pancreatic beta-cells.
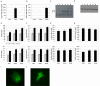
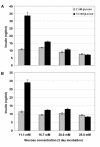
Material and methods: Ten obese diabetic KKAy mice and ten non-diabetic C57BL mice were fed a standard chow diet. After nine weeks, islet RNA was purified and used to measure TIP60 expression. We investigated the effect of TIP60alpha and TIP60beta on glucose-stimulated insulin secretion by transient and stable overexpression in the pancreatic mouse beta-cell line MIN6 and the rat beta-cell line INS-1E.
Results: We found that non-diabetic C57BL mice and diabetic KKAy mice have the same level of both the alpha and beta splice forms of TIP60. Furthermore, we demonstrated that transient and stable expression of TIP60 in INS-1E cells affects neither glucose-stimulated insulin secretion, insulin output nor cell insulin content. Also susceptibility to developing gluco-toxicity was unaffected.
Conclusion: TIP60 over-expression does not affect glucose stimulated insulin secretion, insulin content or abnormal beta-cell function during glucotoxicity.
Figures



References
-
- Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, Levens AR, Yang R, Zhang CY, Lowell BB, Berggren PO, Newgard CB, Bonner-Weir S, Weir G, Spiegelman BM. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev Cell. 2003;5:73–83. - PubMed
-
- Ahren B, Magrum LJ, Havel PJ, Greene SF, Phinney SD, Johnson PR, Stern JS. Augmented insulinotropic action of arachidonic acid through the lipoxygenase pathway in the obese Zucker rat. Obes Res. 2000;8:475–480. - PubMed
-
- Persaud SJ, Muller D, Belin VD, Kitsou-Mylona I, Asare-Anane H, Papadimitriou A, Burns CJ, Huang GC, Amiel SA, Jones PM. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes. 2007;56:197–203. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
