Probing inducible nitric oxide synthase with a pterin-ruthenium(II) sensitizer wire
- PMID: 18085539
- PMCID: PMC3021474
- DOI: 10.1002/anie.200703743
Probing inducible nitric oxide synthase with a pterin-ruthenium(II) sensitizer wire
Figures


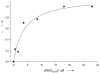

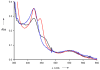

Similar articles
-
Probing heme coordination states of inducible nitric oxide synthase with a ReI(imidazole-alkyl-nitroarginine) sensitizer-wire.J Phys Chem B. 2007 Jun 21;111(24):6628-33. doi: 10.1021/jp071405+. Epub 2007 May 31. J Phys Chem B. 2007. PMID: 17536854 Free PMC article.
-
Sensitizer-linked substrates and ligands: ruthenium probes of cytochrome P450 structure and mechanism.Methods Enzymol. 2002;357:120-33. doi: 10.1016/s0076-6879(02)57672-2. Methods Enzymol. 2002. PMID: 12424904 No abstract available.
-
Proton-coupled electron transfer in ruthenium(II)-pterin complexes: formation of ruthenium-coordinated pterin radicals and their electronic structures.Inorg Chem. 2008 Jan 7;47(1):333-43. doi: 10.1021/ic701759c. Epub 2007 Nov 30. Inorg Chem. 2008. PMID: 18047328
-
Control of redox reactivity of flavin and pterin coenzymes by metal ion coordination and hydrogen bonding.J Biol Inorg Chem. 2008 Mar;13(3):321-33. doi: 10.1007/s00775-008-0343-1. Epub 2008 Feb 13. J Biol Inorg Chem. 2008. PMID: 18270755 Review.
-
Metathesis reactions. General considerations.Curr Top Med Chem. 2005;5(15):1461-72. doi: 10.2174/156802605775009784. Curr Top Med Chem. 2005. PMID: 16378487 Review.
Cited by
-
Functionalised Cofactor Mimics for Interactome Discovery and Beyond.Angew Chem Int Ed Engl. 2022 Jul 18;61(29):e202201136. doi: 10.1002/anie.202201136. Epub 2022 May 31. Angew Chem Int Ed Engl. 2022. PMID: 35286003 Free PMC article. Review.
-
Ru(II)-diimine functionalized metalloproteins: From electron transfer studies to light-driven biocatalysis.Biochim Biophys Acta. 2016 May;1857(5):589-597. doi: 10.1016/j.bbabio.2015.09.004. Epub 2015 Sep 25. Biochim Biophys Acta. 2016. PMID: 26392147 Free PMC article. Review.
-
The Impact of Inorganic Systems and Photoactive Metal Compounds on Cytochrome P450 Enzymes and Metabolism: From Induction to Inhibition.Biomolecules. 2024 Apr 4;14(4):441. doi: 10.3390/biom14040441. Biomolecules. 2024. PMID: 38672458 Free PMC article. Review.
References
-
- Wei CC, Crane BR, Stuehr DJ. Chem Rev. 2003;103:2365–2383. - PubMed
-
- Wilker JJ, Dmochowski IJ, Dawson JH, Winkler JR, Gray HB. Angew Chem. 1999;111:93–96.
- Angew Chem Int Ed. 1999;38:90–92.
- Dunn AR, Dmochowski IJ, Winkler JR, Gray HB. J Am Chem Soc. 2003;125:12450–12456. - PubMed
- Dunn AR, Hays AMA, Goodin DB, Stout CD, Chiu R, Winkler JR, Gray HB. J Am Chem Soc. 2002;124:10254–10255. - PubMed
- Dunn AR, Belliston-Bittner W, Winkler JR, Getzoff ED, Stuehr DJ, Gray HB. J Am Chem Soc. 2005;127:5169–5173. - PubMed
- Belliston-Bittner W, Dunn AR, Nguyen YHL, Stuehr DJ, Winkler JR, Gray HB. J Am Chem Soc. 2005;127:15907–15915. - PubMed
-
- Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, Tainer JA. Science. 1998;279:2121–2126. - PubMed
-
- Raman CS, Li H, Martasek P, Kral V, Masters BSS, Poulos TL. Cell. 1998;95:939–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

