Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers
- PMID: 18085594
- PMCID: PMC3049865
- DOI: 10.1002/cne.21535
Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers
Abstract
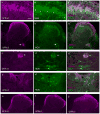
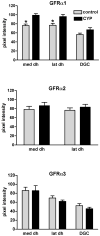
Nerve growth factor has been proposed to mediate many structural and chemical changes in bladder sensory neurons after injury or inflammation. We have examined the expression of receptors for the glial cell line-derived neurotrophic factor (GDNF) family within sensory terminals located in the sacral spinal cord and in bladder-projecting sacral dorsal root ganglion neurons of adult female Sprague-Dawley rats. Nerve fibers immunolabelled for GFRalpha1 (GDNF receptor), GFRalpha2 (neurturin receptor), or GFRalpha3 (artemin receptor) showed distinct distribution patterns in the spinal cord, suggesting separate populations of sensory fibers with different functions: GFRalpha1-labeled fibers were in outer lamina II and the lateral-collateral pathway and associated with autonomic interneurons and preganglionic neurons; GFRalpha2-labeled fibers were only in inner lamina II; GFRalpha3-labeled fibers were in lamina I, the lateral-collateral pathway, and areas surrounding dorsal groups of preganglionic neurons and associated interneurons. Immunofluorescence studies of retrogradely labelled bladder-projecting neurons in sacral dorsal root ganglia showed that approximately 25% expressed GFRalpha1 or GFRalpha3 immunoreactivity, the preferred receptors for GDNF and artemin, respectively. After cyclophosphamide-induced bladder inflammation, fluorescence intensity of GFRalpha1-positive fibers increased within the dorsal horn, but there was no change in the GFRalpha2- or GFRalpha3-positive fibers. These studies have shown that GDNF and artemin may target bladder sensory neurons and potentially mediate plasticity of sacral visceral afferent neurons following inflammation. Our results have also revealed three distinct subpopulations of sensory fibers within the sacral spinal cord, which have not been identified previously using other markers.
Copyright 2007 Wiley-Liss, Inc.
Figures






References
-
- Bennett DLH, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Letts. 1996;206:33–36. - PubMed
-
- Bennett DLH, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

